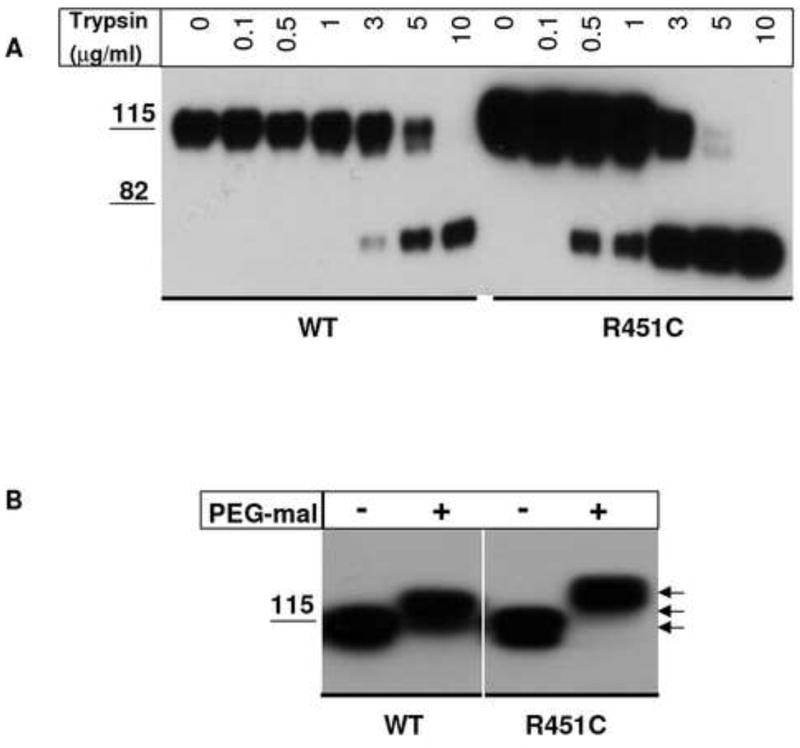
Figure 1. Trypsin sensitivity and PEG-maleimide reaction of NLGN3 wild type and R451C mutant protein.
A) Immunoprecipitated NLGN3 wild type and R451C were treated for 5 min with trypsin at the indicated concentrations and analyzed by SDS PAGE in reducing conditions followed by immunoblotting with an anti-NLGN antibody. Mutant protein is more sensitive to the digestion with trypsin compared to wild type. B) PEG-mal conjugation with free cysteines in NLGN3 wild type and R451C proteins. NLGN3 immunoprecipitated proteins are treated with 1mM PEG5,000-maleimide in denaturing conditions and band shifts are observed on a SDS PAGE followed by immunoblotting with an anti-NLGN antibody. R451C NLGN3 shows a band shift likely corresponding to the alkylation of three cysteine thiols (arrows).