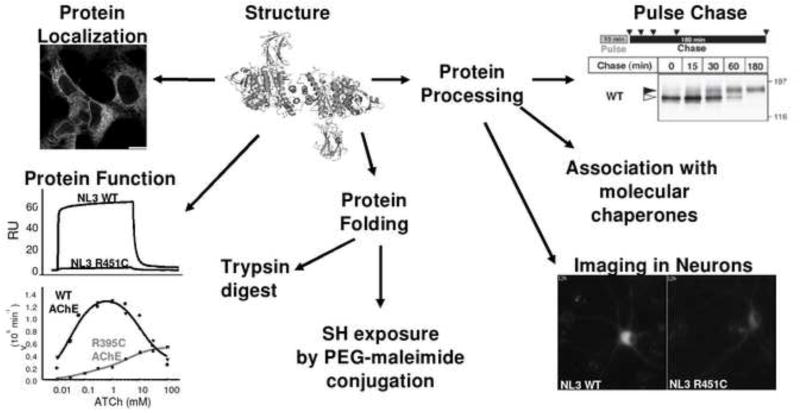
Figure 2. Schematic representation of an experimental approach to study a mutation in the α/β-hydrolase fold proteins.
Determination of mutant protein location with immunofluorescence staining; protein function studies by surface plasmon resonance (NLGNs) or determination of enzyme catalytic constants (ChEs); protein folding by proteolytic digestion with trypsin, and exposure of free cysteine by treatment with PEG-maleimide. Possible alteration of glycosylation processing is studied by sensitivity to glycosidases, pulse-chase metabolic labelling and differential association of wild type and mutant proteins with molecular chaperones during protein biosynthesis. Protein trafficking in the cellular context of the nervous system can be approached using imaging techniques.