Abstract
Dracunculiasis, also named Guinea Worm Disease (GWD), is one of the Neglected Tropical Diseases (NTDs) caused by a parasitic nematode known as Dracunculus medinensis and has been known since antiquity as ‘fiery serpent’ from Israelites. It is transmitted to humans via drinking contaminated water containing infective copepods. Given, its feasibility for eradication, the Guinea Worm Eradication Program (GWEP) was launched in 1980 with the aim of eradicating the disease. Since its inception, GWEP has made an extraordinary progress in interrupting transmission. Globally, the number of reported cases reduced from 3.5 million in 20 countries in 1986 to only 22 cases in 2015 from only four countries namely South Sudan, Mali, Chad and Ethiopia. Since Mali has interrupted transmission of GWD in 2016, currently, the disease remains endemic in only three sub-Saharan African countries namely, South Sudan, Chad and Ethiopia. Each endemic country has its own national Guinea Worm Eradication Program. In Ethiopia, the Ethiopian Dracunculiasis Eradication Program (EDEP) which was established in 1993 has made remarkable move towards interruption of disease transmission and now the endgame is fast approaching. The EDEP with support mainly from The Carter Center, WHO, and UNICEF has reduced GWD by more than 99% from 1994 to 2015. In 2015, only 3 indigenous cases in humans and 14 in animals (13 in dogs and 1 in baboon) were reported. In 2016, 3 human cases, 14 dogs and 2 baboon infections were reported.. Refugee influx from the Republic of South Sudan (RSS), increased animal infections with unknown role in transmission of Dracunculiasis, the presence of hard to reach communities and lack of safe water sources in remote non-village areas remain among important challenges at this final stage of GWD eradication in Ethiopia. This paper reviews progress made towards Guinea Worm Eradication with a focus on the experience of the Ethiopian Dracunculiasis Eradication Program (EDEP), and intervention strategies that need further intensification to realize the endgame. Eradication strategies encompassing community education for behavioral change including raising awareness towards cash reward for reporting Guniea Worm Disease (GWD) and animal infection, case containment, surveillance systems, provision of safe water supply, and ABATE chemical application are discussed. It also summarizes challenges the end game faces and recommendations to strengthen the eradication effort.
Keywords: Guinea Worm Eradication, Endgame, EDEP, Ethiopia
Introduction
Guinea Worm Disease also called Dracunculiasis is caused by a 60-100cm long, filarial nematode worm known as Dracunculus medinensis (1). Dracunculus medinensis is the largest of all human parasitic nematode worms (2,3). Guinea Worm Disease (GWD) is one of the Neglected Tropical Diseases (NTDs) targeted for eradication and it is characterized by a painful skin blister which may ultimately break forming an ulcer (4). Though rarely fatal, it may cause permanent disability and may result in loss of family income and school absenteeism (5,6). Neither anti-helminthic medication nor a vaccine is available to treat or prevent Guinea Worm Disease (2). Hence prevention is the only effective strategy to interrupt transmission of the disease. The disease mainly affects people in rural areas, deprived and isolated communities who depend on open surface water sources such as ponds for drinking. Guinea Worm Disease is transmitted when people in endemic area drink water containing copepods harboring infective larvae.
Once ingested, the copepods (small water crustacean insects) are destroyed by gastric juice in human stomach, releasing the infective larvae. The larvae then migrate to the small intestine, penetrate through the duodenal wall and become adult worms in connective tissues. After about a year, 10-14 months old gravid female worms undergo subcutaneous migration (4). Infected individuals remain asymptomatic until this time. The migrating worms in dermis initiate itchy, inflamed and painful lesions, usually on the lower limb. Eventually, an emerging worm can also incapacitate a person for several months (7,8). The itching and pain prompts the use of water as therapy, whereby infected persons in rural communities often immerse their affected body part into water sources to get relief from the pain. Subsequently, the female worm releases thousands of its immature larvae (L1). These larvae are then ingested by copepods also called cyclops. Cyclops serve as intermediate host for D.medinensis. Several species are known today such as Mesocyclops (M. aequatorialis and M. kieferi), Metacyclops (M. margaretae), and Thermocyclops (T. crassus, T. incisus, T. inopinus, and T. oblongatus (9).
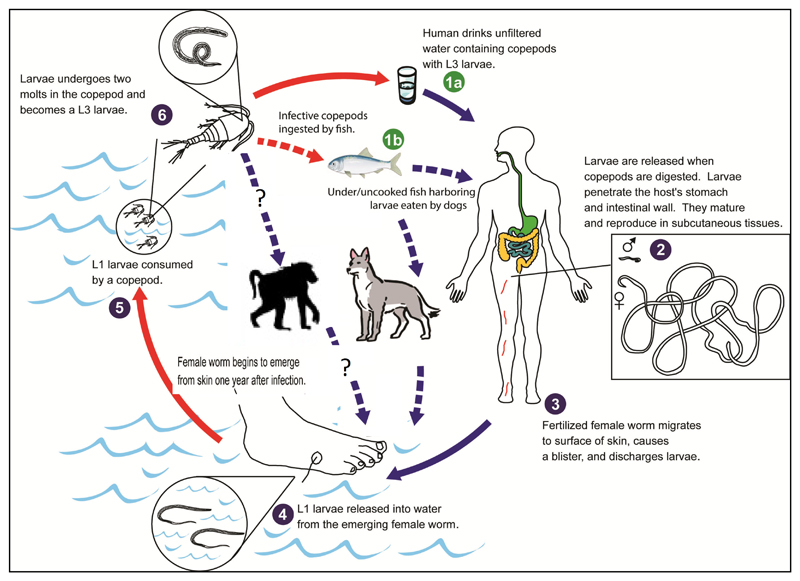
Once ingested, the L1 larvae inside the body of copepods mature to L3 and become infectious to humans. When humans drink water containing copepods which harbor L3 larvae, the infection perpetuates (10). More recently, an alternative life cycle is being proposed which involves dogs and fish (Fig. 1) in Chad. It suggested that dogs are being infected by eating raw entrails of fish which contain viable guinea worm larvae. Humans could be infected by eating raw fish without necessarily drinking unsafe water (11,12). In this proposed alternative life cycle the role of fish as paratenic host in maintaining the L3 larvae and longevity of the infectious L3 is to be investigated. In Ethiopia guinea worm infection is being reported in animals (dogs and baboons) with increasing rate from 2012 to 2016. However, the role of dogs and baboons in the transmission of Dracunculiasis to humans is yet to be determined (Fig 1). While, the understanding of this is crucial, preventive measures are designed based on the lifecycle of the worm and potential reservoir animal hosts and sources of infection to halt the worm’s life at different stages.
Fig 1.
A proposed alternative life cycle of the guinea worm: A challenge for eradication prospects? (modified from reference 27)
Global Guinea worm eradication status
Since, the conception of the Guinea worm eradication program in the 1980s significant progress has been made in reducing the number of cases from 3.5 million (in 1980s) to only 126 cases in 2014 (13,14) and as few as 22 cases by the end of 2015 (15). In 2016, 25 confirmed human GWD cases were reported globally from the 3 remaining endemic countries. Similarly, the number of villages reporting cases had been reduced from 23,735 (in 1993) from 21 endemic countries to 30 villages in 2014 and to only 20 by the end of 2015 (15) confined to only four countries, namely Chad, Ethiopia, Mali, and South Sudan (16). Sixteen (16) out of 20 previously endemic countries had now achieved transmission interruption with Ghana being lately certfied as free of Dracunculiasis by the International Commission for the Certification of Dracunculiasis Eradication (ICCDE) in January, 2015(14). Sudan and Kenya are in the pre-certification stage. Angola and the Democratic Republic of Congo do not have recent history of the diseases. Case importation across countries has also been minimized significantly. Hence, the disease is now brought to the verge of global eradication. However, challenges remain to overcome in the remaining endemic countries in order to realize the end of this painful disease. The risk of case importation, continued low intensity transmission, inaccessibility to endemic localities, emerging infections in dogs and baboons (as in Ethiopia), as well as the changing transmission dynamics with a rising number of dog infections in Chad, insecurities in South Sudan are among other threats to the eradication campaign. Therefore, this paper reviews the current status of the global Guinea worm eradication in Ethiopia and intervention strategies such as surveillance activities, case containment, provision of safe water, awareness creation on GW/cash reward and ABATE application. The remaining challenges and/or gaps in intervention strategies and implications of animal hosts for the eradication efforts is also discussed in brief.
Methods
Published papers related to infectious diseases elimination, particularly of GWD were extracted from PubMed, WHO, CDC, and Google Scholar. Of these, the CDC website, WHO online atlas (GW data store) and recent data from EDEP were extensively used. The ‘AND’ search function, by which search terms can be combined to narrow down the results was also employed. Overall, 80 results for the keywords “Guinea worm eradication” and “Ethiopia” were obtained of which 70 pertinent to this study and 63 were included in the review. Moreover, data obtained from personal communications with the national GWD experts from Federal Ministry of Health and Ethiopian Public Health Institute (EPHI) of Ethiopia were also included.
Results
Guinea Worm Eradication in Ethiopia
After 22 years of battle against GWD, Ethiopia has remained one of the Guinea worm endemic countries. Historically, the first case of Guinea worm was reported in 1969 (17) far before the launch of the guinea worm program in Ethiopia. Guinea Worm Disease has been endemic in Gambella region and South Omo Zone of SNNP Region in the past but indigenous transmission in South Omo has been interrupted as of 2001, leaving Gambella region as the only indigenous transmission area since then (18). In Gambella region only one district (Gog) is currently endemic for the disease where low intensity transmission is ongoing. All the remaining districts in Gambella except Godere and two districts in SNNP Region (Nyangatom and Surma) are classified as high risk areas in Ethiopia as most of them except Surma were formerly endemic plus there is population movement within these districts. Furthermore, some of the districts in Gambella such as Lare, Wantoa and Jikawo are points of entry for refugees. For instance, the districts Itang, Lare, Gog, Gambella Zuria and Dimma are currently hosting a refugee population of more than 270,000. In the case of Surma and Nyangatom districts, there is cross border population movement related to trade or market which may increase the risk of importation of GW cases from Eastern Equatorial State of South Sudan (19).
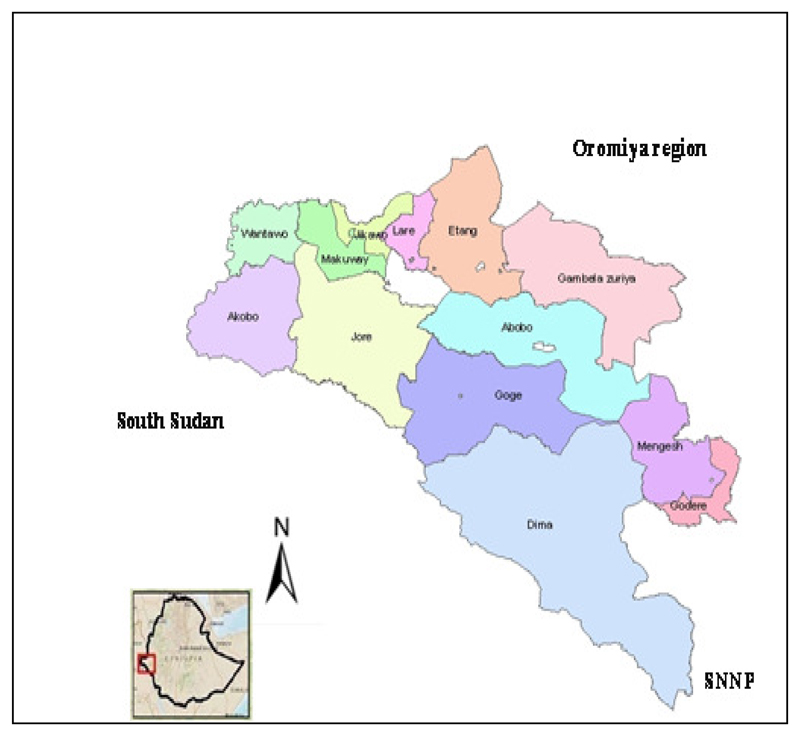
Gambella, is one of the 9 regional states of Ethiopia located 766 KMs southwest of Addis Ababa, the capital of Ethiopia. It lies between 7-8° North longitude and 33-35° East latitude and share borders with Oromiya to the North and East, SNNPR to the South, and the country of South Sudan to the west (Fig 2). The altitude of the region ranges from 300-2300 meters above sea level with an average annual rainfall between 800 to 2100mm and mean annual T° of 30.7°C (20).
Fig 2.
Gambella Regional State administrative map, 2015
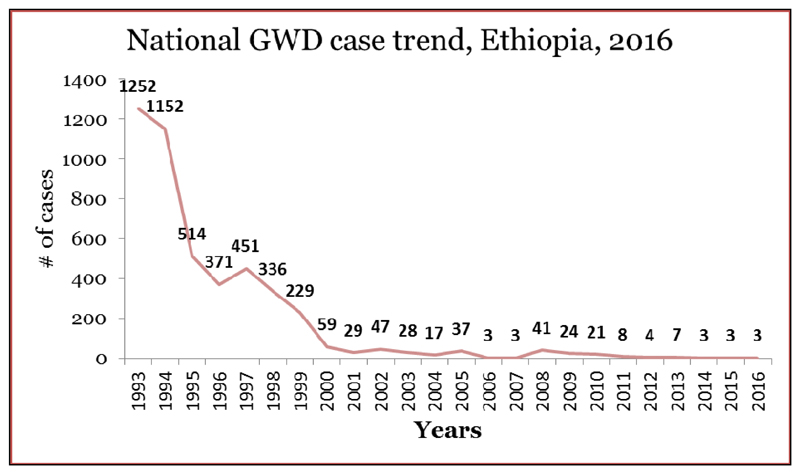
The Carter Center (TCC), WHO and UNICEF are the key partners supporting the EDEP. Through active leadership of Ministry of Health/EPHI and Gambella Regional Health Bureau and with the support from WHO UNICEF and TCC, EDEP has made a significant progress towards transmission interruption since the commencement of the eradication effort in 1993. Surveillance, awareness creation of cash rewards, documentation, reactivation of the national certification committee (NCC), establishment of villages under active surveillance (VAS) and detection and reporting of rumors are some of the supports given to EDEP (19). There were 1,129 reported cases of GWD in a total of 113 endemic villages in 1993 and 1,252 in 1994 (14). Since then the number of cases declined significantly and then a low level of transmission was maintained for long period of time from 2001 till end of 2016. In humans, the number of cases has reduced from 1,252 in 1994 (21) to only 3 cases in 2015 and 2016 (15) (Fig 3).
Fig 3.
Reported cases of Guinea worm disease in Ethiopia from 1993 to 2016. Data source: Synthesized from WHO online data base (22)
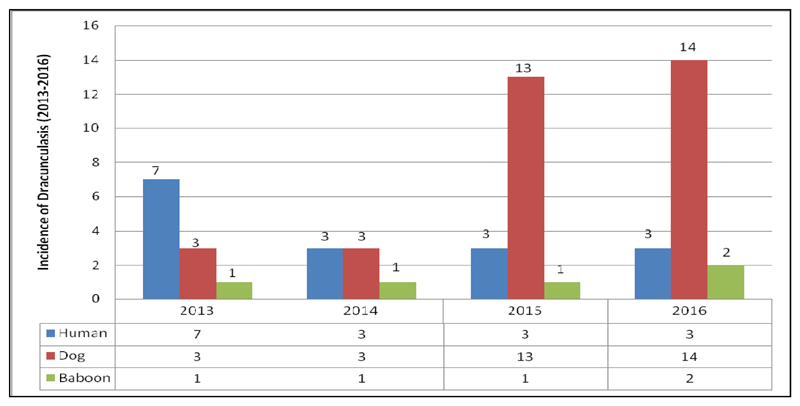
EDEP started to document GWD infection among animals since 2013. More, recently it appears that infection in dogs is spreading fast. Three infected dogs and one baboon were reported both in 2013 and 2014. A total of 14 animal infections (13 from dogs and 1 from baboon were reported) in 2015. Furthermore, 16 animal infections (14 dogs and 2 baboons) were reported in 2016 (Fig 4). Eight out of the 11 dog infections and 1 of 2 baboon reported in 2016 are yet to be lab confirmed. All human and animal infections reported in 2016 between Jan-Sep occurred in Gog district of Gambella region except 1 dog infection reported in Abobo. Similarly, all of the GW cases reported in humans and animal infections in the year 2015 occurred in Gog district of Gambella region (14) except one human case which was reported in Abobo district. Low intensity transmission still continued in Gambella region. Uncertainty in source of transmission, unclear transmission dynamics, low involvement of health extension workers (HEWs), population movement across the Republic of South Sudan, poor infrastructure, difficulty in containing dogs, poor access to remote areas, breakage of boreholes and lack of safe water sources as well as unsuccessful drilling attempts in some villages are some of the challenges faced by EDEP.
Fig 4.
Trends in Guinea Worm cases in humans and infection in animals, Ethiopia (2013-2016)
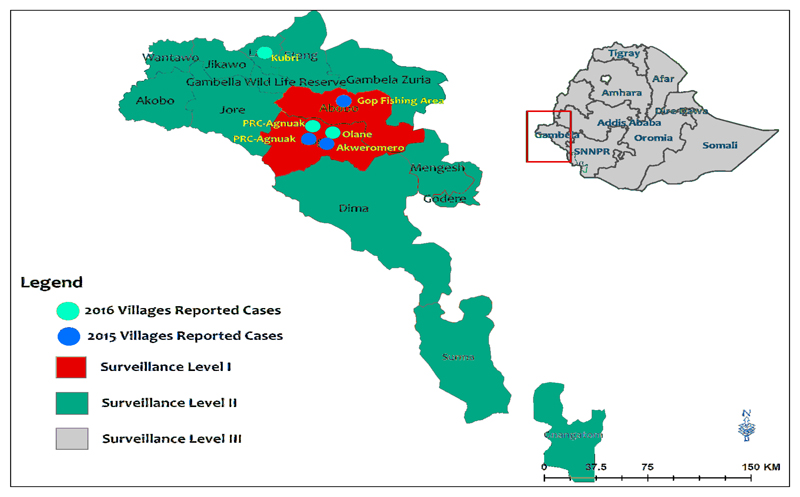
The rising number of Dracunculiasis infections in animals especially dogs might impose a challenge to the EDEP. Operational research needs to be done to understand the transmission dynamics. Though, most of the cases are indigenous, there has been imported cases of GWD as well. For instance, there was one imported case in the Elia Kebele of Itang Special District (Gambella district) during 2013 probably due to cases being left uncontained in Batpullo in 2012 (18). There is also a case reported from Lare district of Gambella region in 2016 who is most likely imported from South Sudan. Though the risk of receiving imported cases from South Sudan is reduced as a result of sharp decline in the incidence of GW cases in South Sudan, it is still a threat because of large number of refugee influx. However, cross border communication and surveillance at border areas needs to be strengthened. Districts at risk of receiving imported cases are indicated by yellow in the figure below (Fig 5). Many of these areas have refugee entry points for migrants coming from South Sudan.
Fig 5.
Guinea worm levels of surveillance and villages reporting
Case studies related to unknown sources of infection
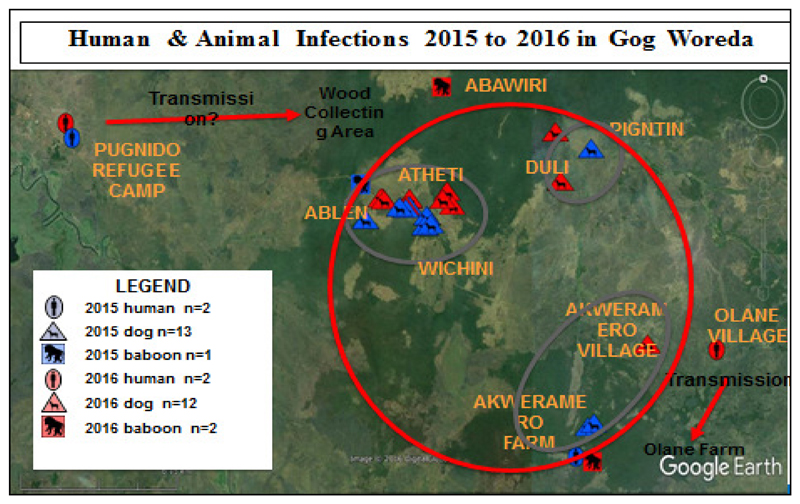
One case of GWD in 2012 and one in 2014 were reported outside of the Ethiopian Guinea worm disease ‘transmission season’ and their sources of infection are not yet known. For the 2014 case, transmission was likely occurred in Which in Village linked to possible contamination of Tanchay or Odale ponds (common sources of infection) from possibly missed cases in 2013. At the same time there could be a possibility of contamination from Okem (the case reported in Gog in 2013) if the active surveillance during the period (2011-2013) was tight enough to detect every new case of GWD. A final possibility is that contamination occurred from dogs. However, these hypotheses are only suggestive but not conclusive. (23). In 2015, almost all human and animal cases occurred in a small area of Gog district of Gambella region, which is suggestive that GW cases are becoming confined to a small area compared to the case distribution in the previous years. Three main areas of infections in 2015 were: 10 dogs and 1 baboon GW infection from the Atheti/Ablen/Wichini cluster, 2 dog infections and 3 human cases from the Akweromero Farm cluster, 1 dog infection from the remote an area with no history of human or animal infections. In 2016 the two human cases were from PRC Anguak and Olane village of Gog district, respectively where infection is likely linked to Abawari village which is close to Atheti/Ablen/Wichini cluster and Olane farm that is also nearby Akweremero farm cluster. In these clusters, transmission is likely going on as they are reporting most of the 2015 and 2016 animal infections. (Fig 6).
Fig 6.
Human and animal infections in 2015 and 2016 in Gog Woreda, Gambella, Ethiopia, 2016
Review of GWD eradication strategies
Experience of Ethiopia
GWD is an easily preventable disease with effective intervention strategies such as provision of safe water and distribution of filters to residents in endemic communities. The key intervention activities that are of paramount importance to the Guinea Worm Eradication Program in general include: Provision of safe drinking water, surveillance to monitor case occurrence and intervention activities, health education for behavioral change, application of ABATE chemical to water bodies suspected of contamination with copepods, filtering potentially contaminated water, containment of transmission through isolation of each patient to prevent contamination of drinking water sources, and manual extraction of the worm (24–26). Owing to the fact that the number of GWD cases declined to a historically low prevalence the diseases is on the verge of global eradication. There is a need to strengthen and sustain eradication efforts as much as ever. As the world is now moving towards a final push to eradicate the disease, the remaining endemic countries need to intensify the existing time tested strategies and identify innovative strategies and respond to the emerging challenges in order to meet and pass through the strict certification process. Regular monitoring of the disease occurrence through prompt rumor investigations, increasing/revising cash rewards as well as creating awareness of reward needs to be intensified than ever before. Moreover, prevention strategies to address emerging challenges such as increased infection of dogs and baboons (in Ethiopia) and domestic dogs and cats (in Chad) (27) shall be devised and implemented accordingly.
In Ethiopia, heightened case search and active surveillance, awareness creation towards cash reward for reporting both human and animal cases and self reporting, early rumor investigation, case containment and management, provision of safe water supply, ABATE application to water sources, filter distribution are among key activities of EDEP to eradicate GWD. Other activities such as improving GWD documentation, supportive supervision, awareness level assessment are recently under active implementation to cross the finish line of GWD eradication in Ethiopia. The recent achievements in the EDEP’s eradication strategies also include the strengthening of refugee surveillance, intensification of intervention efforts in forest areas, hunting places, fishing camps and non-village settlement areas (28). Following an outbreak of animal infections seen in recent years, particularly in Gog district, the EDEP has also launched cash reward system for individuals reporting animal cases (28). Chaining dogs before emergence of the worm to ensure appropriate containment and investigation of transmission through dog house-hold surveys are being implemented in Gog endemic district (29).
Safe water supply
Due to its association with consumption of unsafe drinking water, GWD eradication can be achieved by provision of safe water alone (30). Provision of safe drinking water has shown significant impact by reducing incidence of GW cases (31–34). While safe water is a key component to interrupt transmission of GWD (32), access to villages in remote areas and mobile populations remain a big challenge in endemic countries. Even the latest reports from the WHO show lack of safe drinking water in a number of villages where transmission is still ongoing. For instance, only 42 out of 70 villages (60%) had safe drinking water in Gog district of Gambella region where all animal cases and 2 of 3 human cases were reported in 2015 (29). However, coverage has not been a problem in many areas. For instance, breakage of boreholes was reported to be common in South Sudan, where 42% of endemic villages were found with one or more functional boreholes in 2014 (16). It is therefore critical to improve provision and accessibility of water supplies through regular check for functionality of boreholes.
Protecting already available surface waters from contamination by infected individuals or avoiding drinking such water sources, as well as protecting wells and filtration (24), and construction of borehole wells (35) can be employed to ensure safety of drinking water. Investing in safe water supply is shown to accelerate eradication. Ghana realized an expedited eradication by achieving 84% population access to safe water (36). However, safe drinking water may not be accessible to all including those hard to reach communities in remote areas. Different types of water filters have been developed with varying acceptability to community. In South Sudan nylon filters were better accepted and efficiency employed than cotton cloth filters. Life straw filters are also available. Low depth water sources limit the use of Life straw filters (37). So, improving filters to meet communal needs is highly recommended. Infiltration systems, machine-drilled boreholes, hand-drilled tube wells, community sand filters, roof rain catchments, and ground rain catchments have been implemented to improve safety of drinking water in Ethiopia. Moreover, monofilament cloth filters have been provided for households and pipe filters to highly mobile population segments such as hunters, shepherds and travelers (38).
While potable water supply is an essential component of GW intervention strategy, there is still a problem of proper usage in some cases, even while the coverage is high. This could be due to remoteness of water sources to people’s homes among other factors (2). Therefore, authorities and programs should give priority to high risk localities and endemic communities in remote rural areas. Aside from provision of safe water supply, there is also a need to provide intensive health education in order to ensure adherence to utilization of such measures. Sustainable safe water supply to endemic communities in Ethiopia remains problematic. Unsuccessful drilling attempts in some areas of Gambella, low access to safe water supply in Akobo district, and unavailability of data on safe water supply coverage despite installation of a limited number of hand pumps have been noted in Ethiopia. (28)
Surveillance
For the eradication of Guinea Worm Disease, application of highly sensitive and validated diagnostics and effective control measures alone might not be sufficient. Regular surveillance-response approaches to monitor sustained progress is also required (39). Unlike control programs, which could be achieved by sampling or sentinel surveillance, eradication programs generally need extremely sensitive and rigorous surveillance systems. This is because they have to detect all cases including those imported from the neighboring countries (24) as well as in between counties in the same nation. Improving surveillance systems to detect all cases and report all rumors, become extremely more important than ever to follow decline of diseases incidence. The intensity of implementing active surveillance greatly impacts the speed of elimination of a particular disease. In this regard, the identification and training of village based health workers and village volunteers in each endemic area is critical to reach all communities.
In Pakistan a village implementer per each village and in Ghana a resident volunteer had been instituted and trained to undertake an active case search in endemic villages (24) and provide reports on monthly basis. Case definition is another area of improving active surveillance schemes. Case definition adopted by eradication programs should comply with standard definitions to facilitate detection and reporting. Improving accuracy, coverage and intensifying the whole arena of surveillance strategies is highly urgent. If surveillance is not accurate and complete, cases may be missed. In such conditions it is obvious that zero case reports would be misleading and make countries claim transmission interruption while in fact diseases transmission is ongoing. High rate of workforce turnover and low level of competency have been reported in South Sudan (37) in addition to incomplete reports.
In Mali, insecurity had led to interruption of eradication activities in many parts and forced surveillance reports available only from southern regions especially during the 2012 coup d’état. Currently, cash rewards have been raised to 100 USDs in all four remaining endemic countries (Chad, Ethiopia and South Sudan) (16) to individuals voluntarily reporting each case. Such a heightened surveillance should be enforced and sustained to achieve global eradication in near future. Surveillance is not a single step process that stops as an endemic country achieves transmission interruption. It should rather continue for a reasonable period of time even after transmission has been interrupted in order to verify if interruption of transmission is maintained for at least 3 years before certification. Given, the long duration of incubation during which the patient is unaware of infection and can travel far distances, programs are forced to identify all communities within an endemic transmission area, high risk adjacent non-endemic communities, as well as localities where residents travel. Therefore, it is recommended to devise a three level surveillance system (Level I, II and III) where all rumors shall be investigated within 24 hours and reporting rates monitored monthly. Level I surveillance is meant for endemic communities, level II adjacent high risk non-endemic communities and level III all other communities in non-endemic districts not at high risk of importation of cases (12).
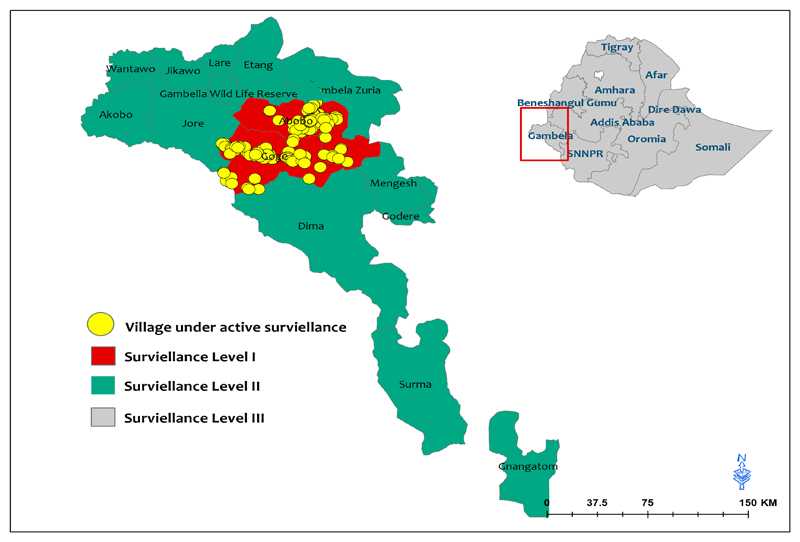
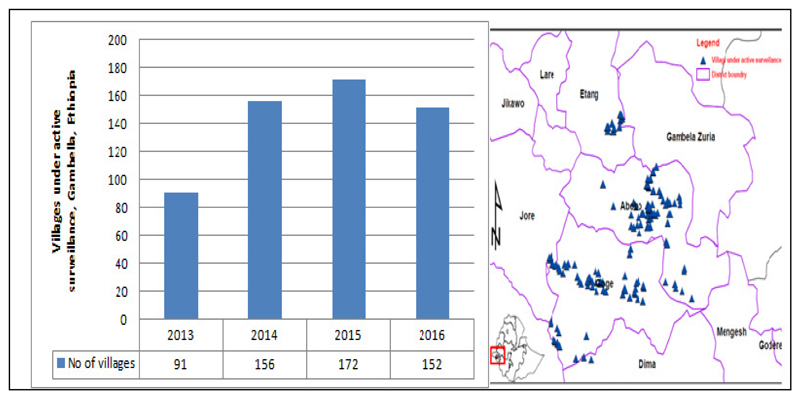
A monthly monitoring of the rumor reporting rates, as well as reward awareness level is essential additive to active house to house search for cases. In 2015, there were 172 villages under Level I surveillance/active surveillance (VAS) (Fig 7,8) in three districts of Gambella: namely, Gog, Abobo and Itangas well as 12 districts under surveillance level II (2 in SNNPR and 10 in Gambella) supported by The Carter Center. However in 2016, only two districts (Gog and Abobo) were put under surveillance level I (152 villages are under active surveillance) Fig 7. To heighten surveillance, village based volunteers (VBVs) are being trained and deployed to all VAS.
Fig 7.
Levels of GWD surveillance in Ethiopia, 2016
Fig 8.
Villages under active surveillance of GWD, EDEP, Ethiopia (2013-2016). Average number of VAS increased from 91 in 2013 to 172 in 2015(28)
In countries like Ethiopia case importation from the Republic of South Sudan has been a risk and remained a challenge for the endgame as the two countries share a long border. For instance in Surma and Nyangatom districts in SNNP, there are cross border population movements related to trade which may lead to high risk of importation from Eastern Equatorial State of South Sudan. There are four major entry points of South Sudanese refugees to Ethiopia, and 6 refugee camps in Gambella region comprising a population of 236,045 refuges in 2015.
Though there is an overall improvement in the surveillance system, further intensification of surveillance activities including awareness assessment in refugee camps and non-village settlement areas are needed. Documentation of Guinea worm data has been weak across the endemic region. But very recently, documentations of GWD is greatly improved at the regional and district levels (personal communications). This has been mainly due to support from WHO and TCC provided to EDEP. However, there remain needs to ensure appropriate and consistent documentation of GWD rumors, from community to national level at all level particularly in non-endemic areas of the country. Monitoring of records archived properly and bill boards are in place is also critical. The completeness and timely report of surveillance data has been greatly improved in 2016, compared to 2015. For instance in some endemic districts such as Abobo completeness reached 86% and timeliness 81% (28). This is far better compared to data completeness reported in South Sudan (ranging from 11% to 40%) (37). To further increase the rate of timely surveillance data report and completeness, training and supportive supervision needs to be strengthened.
Case containment
Surveillance aimed to detect and report cases including the last case should be followed by rigorous containment and management of every case. Case containment is one of the hall-marks of an effective eradication program. According to WHO/CDC criteria (41) a standard Dacunculiasis case containment shall fulfill the following criteria: detection of cases within 24 hours of worm emergence, the patient had not entered any water source since worm emergence, the patient has been meticulously managed by cleaning and bandaging until the worm is fully removed, and by giving health education to discourage the patient from contaminating any water source (if two or more Guinea worms are present, the case is not contained until the last worm is pulled out), and Abate is used if there is any uncertainty about contamination of sources of drinking water, or if the source of drinking water is known to have been contaminated (12).
Given the fact that successful case containment demands the above criteria including supervision, to ensure that all cases are reported and appropriate measures are taken, it is essential to note that it is costlier compared to traditional measures. Case containment leads to the protection of water sources from becoming contaminated by infected patients. By doing so it protects the community at large and prevents ongoing transmission. As has been noted in WHO reports, a significant number of GWD cases were left uncontained in remaining endemic countries. In Ethiopia 2 of 3 met the case containment criteria in 2014 alone (11). However, containment rate improved significantly since 2012. In 2015, 100% of the cases in humans were contained (28). In contrast to human cases, containment in animals has been poor during the same year.
Ethiopia reported 14 infected animals but only 4 (28.6%) were contained (15). However, in 2016 containment for dogs were greatly improved. Hence, improvements in case containment and management of animal infections remains important areas to work on. Establishing case containment centers in known endemic districts (42), and treating individuals as ‘inpatients’ at containment centers with provision of all necessary needs including incentives have been implemented to improve compliance with case containment criteria. However, as the case load declines containment should become extremely rigorous to ensure that all detected cases and animal infections are contained, including the last case if the disease has to be eradicated.
Case management
Given dracunculiasis has no effective medication, treating the patient relies on removing the worm by rolling it using a stick which has been the practice since 1550 BC. The extraction has to be done by skillful health care practitioners. Case management helps to avoid the pain due to the emerging worm as well as to prevent contamination of water sources. Breakage of worms during manual extraction is not uncommon. In Ghana a high worm breakage rate (46%) was reported and when that occurred it was associated with worsening inflammation and likelihood of secondary bacterial infection and disability (43). In such cases wound management is necessary. Patients might also benefit from analgesics to reduce inflammation and antibiotic ointment to prevent secondary bacterial infection at the wound site. Moreover, a controlled submersion of the affected area into a bucket of water that cause discharging of larvae by female worms making it less infectious also confers relief from the burning sensation. The use of bandaging, to prevent the victim from immersing the affected part to water sources (2) has also been widely used and shown to promote early self-reporting of cases. These techniques are also equally recommended for application to dog worms.
Vector control
Vector control involves the application at a monthly interval of organophosphate insecticides such as ABATE (temephos) to surface sources of drinking water to kill cyclops (44–46). As reviewed by Awofeso, when used at optimum dose it is safe to mammals including human beings and shows low level of bioaccumulation tendency (37). The appropriate dose of ABATE, identification and calculation of volume of potentially unsafe water sources that need treatment are critical to the efficacy of this method. If inadequate dose is applied, ABATE will not kill cyclops (2), and rather they could develop insecticide resistance. The risk of missing drying ponds which harbor infected cyclops (47), large pond sizes and difficulty in reaching water sources in remote areas (37) remain challenges with ABATE application. Unless all water sources suspected to harbor copepods are treated, transmission is likely to perpetuate delaying eradication. As it is expensive and technically difficult to reach large ponds, application should be made only selectively in affected areas during peak transmission seasons.
It has been shown that socio-cultural factors may influence the acceptance of ABATE application in certain societies. The implementation of ABATE application at the moment is promising in currently endemic areas, however. In Ethiopia, all suspected water sources received regular ABATE application during 2014 and 2015 (14,15). However, the mapping and application of ABATE chemicals to reach all the possible hidden water sources in the non-village settlement areas, such as farming, honey collection forests, hunting places, fishing camps and stick collection sites needs also to be considered in all high risk villages. There is no evidence that ABATE application has not been acceptable by the endemic communities. Therefore, application of ABATE regularly to all suspected water sources is critical. All potentially contaminated water sources both from humans and dogs need appropriate ABATE application. However, it is difficult to apply ABATE all over large water bodies, such as lakes connected to Alwero River in Ethiopia (28). Hence, pond fencing by making use of cloth barriers (Fig 10) would be important before application of ABATE larvicide to smaller partitioned sections of the lagoons (14).
Fig 10.
Fencing ponds for effective Abate Application
Education and Behavioral change
Health education is an essential component of Guinea Worm eradication campaign. It is directed towards ensuring behavioral change and adoption of preventive practices by individuals and community at large (1). It includes educating communities to use safe water sources, teaching patients not to immerse their affected body parts into water sources while the worm is emerging, show how to filter water to remove copepods, creating awareness on reward systems to encourage voluntary case reporting and containment (48,49) as well as how to apply ABATE larvicides (50). Community education has been prioritized at the very top of the eradication strategies by some authorities given it is the community at risk who should take the primary responsibility if successful interruption of transmission has to be achieved in a particular locality. The tragedy is not the fact that Guinea worm exists; rather it is that impoverished communities have not yet been informed how to break the life cycle of the disease.
Villagers and communities can be reached and information disseminated either in person such as via house-to-house visit, market places and schools by GWEP volunteers or through electronic mass media or posters. Awareness creation to community through a series of films and drama has been also practiced. For example ‘Foul Water Fiery Serpent’ in 2010 and ‘Heightened Surveillance’ in 2012 (51,52) were disseminated. To mention the experiences of previously endemic countries, health education especially by Village-Based Health Workers (VBHWs) has been shown to highly impact the prevalence of Dracunculiasis. In Nigeria up to 88% reduction was attributed to community education. In Ghana, face to face health education was shown to have led communities to buy filters (53–55). If transmission has to be interrupted, community based education shall be reached to all residents in endemic areas to ensure that no water source is contaminated and that filters are appropriately used (56). Educating people is generally a key strategy in eradication efforts (57), as all other intervention strategies demand community awareness and behavioral change. Intensification of community education is now required in the remaining endemic countries including Ethiopia.
There are promising experiences of such heightened community education strategies in Ethiopia. For instance, given the low level of awareness on GW in areas other than endemic and formerly endemic communities in Ethiopia, a program called “communication for behavioral impact” (COMBI) has been developed to the EDEP with the help of WHO and international consultants and the plan was partly implemented in 2015 and 2016. COMBI is aimed at creating heightened awareness on Guinea worm and cash reward at national levels with a special focus on endemic districts in Gambella Region and high risk areas in the South Omo and Bench Maji zones. The suggested theme of this program is “Looking for the Last Guinea Worm” (LLGW). It is an all inclusive intense plan involving the community mobilization (through meetings and dialogue in each village with community members and responsible staffs from MOH, the TCC staff and Regional to village bases health workers), “personal sellers” where by village health volunteers (VHV), Health Extension Workers (HEWs), Village/Kebele Leader of the Women’s Health Development Army (WHDA) shall undertake a house-to-house visit in every household in endemic districts and high risk areas to disseminate information of the LLGW/GW award.
Moreover administrative mobilization of all staff in the Ministry of Health (MOH) at the village or Kebele level, media promotion (through radio-TV talk, press conferences, facebook, twitter, text message), advertisement via MRIP (Massive, Repetitive, Intense, and Persistent) Radio-TV- Newspaper advertising campaign, point of service promotion (placing posters in all facilities, local pharmacies, schools, shops, and business partnerships for the LLGW/GW award are the approaches for the implementation of COMBI. So it is expected to address any communication gaps that were observed in the previous years and hence could bring about a significant impact on behavior at a national level and high level awareness of the GWD/cash reward in the endemic and high risk regions in particular. With such a behavioral campaign, EDEP is trying to fully raise awareness of GWD/cash reward. However, health education and behavioral change are not without challenges. For instance, in South Sudan, villagers believe that Guinea worm is transmitted by eating ‘spoiled food’. Similarly traditional healers consider it as protruding nerve (58). Thus successful community education should triumph over cultural, behavioral and attitudinal challenges in different societies. Therefore, it is essential to understand local beliefs of residents prior to approaching such societies.
Moreover, the seemingly changing transmission dynamics of Dracunculaisis in countries such as Chad and increasing animal infections in recent years in Ethiopia warrant a need to devise specific interventional approaches targeting animals. Infection of dogs eating entrails of fish and humans eating undercooked fish in Chad (59) forced GWEP to begin new educational messages by 2013. Such messages for villagers include preventing dogs from eating raw fish entrails and tethering infected dogs until the worm is removed, inform villagers to sufficiently cook, dry and or smoke fish before eating. Educating community to report cases both in humans and dogs should also became part of the intervention in Chad and Ethiopia as well. Given, an outbreak of animal infections in Ethiopia since 2013, raising the community awareness about the issue and cash reward for reporting animal cases has become part of activities put forth for Guinea worm eradication. EDEP introduced cash reward system for reporting GWD in animals in 2015 (28). Education on these aspects and the possibility of water contamination by infected animals is important to improve the overall awareness of endemic communities as well as in high risk and non-endemic areas. It has become important to extend awareness raising activities in non village areas and refugee camps. In order to assess impact of the interventions awareness surveys have to be done on an ongoing basis and actions taken based on findings of the surveys.
Discussion
Given a substantial reduction in the annual incidence of GWD cases, it seems eradication would be realistic by 2020. However, there is still a need for heighten interventions. It is clear that such an end phase of eradication program demands extensive effort to search for last GWD cases, as well as to trace all infection sources. Moreover, such heightened intervention should be maintained for a reasonable period of time (at least three years) after transmission interruption to make sure that the re-establishment of the disease is unlikely. An in-depth investigation of all infection sources and explanation why some GWD cases remain uncontained become critical. Currently, the key challenges in Ethiopia include unknown source of infections that would have led to persistent low level ongoing transmission in remote areas, high rate of population movement and, intermittent insecurities in Gambella region. The rising number of infected dogs in Ethiopia between 2013 and 2015 has become concerning and needs further investigation. To effectively fight GWD, case containment in dogs should be devised as well. In order to understand the role of fish in the Guinea worm life cycle, the longevity of L3 larvae should be further explored which might help discover new intervention strategies towards fish species. Timely containment of infected dogs, burying fish entrails to avoid dogs from becoming infected and rewarding individuals for reporting GW infections in dogs. Educating people to report rumors related to dog infection, and creating awareness of cash rewards should also be part of GWD eradication interventions.
In Ethiopia there has been a long delay in stopping transmission of D. medinensis. This might be due to the fact that cases of GWD have been rela-tively low in number and occurred among remote communities in a region with particularly weak surveillance at the peripheral health service. Population mobility across the Ethio-South Sudanese border increases risk of case importation. The risk is minimal currently because of the significant reduction in the number of GWD cases reported by South Sudan. Whatever the case might be, cross border collaboration and monitoring of migrants’ flow should be strictly considered by the EDEP. Moreover, lack of safe water sources due to incomplete coverage particularly to populations living in remote areas, broken boreholes, and unsuccessful drilling attempts in some endemic villages such as Winchini and Ablen (Personal communication) have been a challenge for EDEP previously. GWEP implementing partners and donors should all advocate to the Government of Ethiopia to sustain the highest level of political commitment to eradicate the disease.
In Ethiopia a poor surveillance system had been believed to have led to low intensity transmission persisting in Gambella Region to date (28). Unclear sources of GW transmission have been a great challenge as well. Therefore, we generally suggest if the scientific community could work towards identification of infection sources and inform EDEP for evidence based intervention. Mapping of hidden water sources in all at risk villages, expanding ABATE chemical application in all at risk villages, extending the GWD intervention activities outside traditional settlement areas (such as in refugee camps), empowering health extension workers to improve their commitment, heightened community awareness on reward and GWD, and reporting and immediate investigation of all rumors should be implemented to facilitate GWD eradication in Ethiopia. Lastly, we recommend evaluation of the existing surveillance system to make it robust enough to capture all new cases throughout the country. Furthermore, operational research towards the transmission dynamics of GWD in dogs and baboons should be undertaken to determine if they have a significant role in the GWD life cycle. Collaboration especially between Ethiopia and Kenya, work with the military to strengthen surveillance for Guinea Worm Disease in conflict affected areas, capacity building in integrated community-based surveillance at the State, County, Payam and Boma Level and improve funding and logistic support for the programme would greatly enhance the eradication efforts.
In the face of the aforementioned challenges, however, the global Guinea Worm Eradication Program has made substantial progress towards eradication. Dracunculiasis eradication is thus to be realized without a vaccine or drug (60) in the immediate future. The last few cases are generally the most difficult to find as they are located in hard to reach or inaccessible foci in endemic areas and may include asymptomatic cases (61). Failure to detect and contain the last cases is most costly as it prolongs the endgame possibly resulting in fatigue of eradication effort (62) as well as seeding later outbreaks(63). Therefore, strengthening the lessons learned from previous eradication experiences such as sustained political commitment and high level advocacy initiated by the former US president Jimmy Carter, remain mandatory until GWD is no more a public health concern.
Strengthening national awareness towards cash reward, responding in a timely manner to every rumour and containing every emerging worm remain the key elements to ensure heightened surveillance. It will require a full resolve of all the affected countries, supporting partners, and endemic communities to make an all-out effort with zero tolerance for any slippage in order to achieve eradication in very near future. The best use of all opportunities with intensive and sustained surveillance in Guinea worm-free areas to prevent any re-establishment of new foci of transmission is highly recommended. The existing support and solidarity of partner organizations such as the WHO/CDC, UNICEF and the Carter Center (64), and operational research from the scientific community need to be in place in order to overcome the myriad of challenges and make a final effort to end the disease. Intensification of all the existing intervention strategies, including heighten surveillance, increasing cash rewards, early rumor investigation, community education for behavioral change, abate application to potentially infected water sources, filter utilization and operational research to address unanswered and emerging question need to be addressed.
Conclusion
The global burden of GWD has fallen by more than 99% by the end of 2016. Only 25 confirmed cases of GWD were reported in 2016 from three endemic countries namely, South Sudan, Chad and Ethiopia. In Ethiopia, the disease remains endemic in few villages of Gog District of Gambella region. It is remarkable that since its commencement of active case search in 1993-1994 the Ethiopian Dracunculiasis Eradication Program (EDEP) has made significant progress towards elimination of GWD. The EDEP reported only 3 human cases and 14 animal infections (13 in dogs and 1 in baboon) in 2015 and only 3 cases in humans, 14 in dogs and 2 among baboon in 2016. With recent enhanced political commitment to eradicate the disease by the Government of Ethiopia as well as substantial financial and technical support by the Carter Center, WHO, EPHI and Gambella RHB, more can be done to improve Guinea worm surveillance and case management, provide better quality community education and expansion of potable, water supplies both in Guinea worm endemic villages, high risk communities and non-village areas. The speed of interruption of transmission depends on sustained implementation of heightened active surveillance in all areas including endemic, non endemic high risk and never endemic areas. Raising nationwide public awareness for rewards for reporting both human cases and animal infections, community mobilization and engagement, enhanced supportive supervision, sustained political commitment and high level advocacy, appropriate record keeping and documentation, as well as addressing issues related to sources of infection in animals have to be further intensified. Active, sustained surveillance in Guinea worm free areas has to continue to prevent any re-establishment of new foci of transmission as well. As tough as the journey to GWD eradication is, the EDEP is ever committed to end the game and attain the global target and join list of countries that eliminated GWD and contribute its share to global effort to eradicate the disease.
Fig 9.
A partial view of photos: Guinea worm cases in animals reported in 2015 in Gog district of Gambella region, Ethiopia
Acknowledgments
KD is funded by Wellcome Trust Intermediate Fellowship in Public Health and Tropical Medicine [grant number 201900].
References
- 1.Biswas G, Sankara DP, Agua-Agum J, Maiga A. Dracunculiasis (guinea worm disease): eradication without a drug or a vaccine. Philos Trans R Soc Lond B Biol Sci. 2013;368(1623) doi: 10.1098/rstb.2012.0146. 20120146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cairncross S, Muller R, Z N. Dracunculiasis (Guinea worm disease) and the eradication initiative. Clin Microbiol. 2002;15:223–46. doi: 10.1128/CMR.15.2.223-246.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruiz-Tiben E, H D. Dracunculiasis (Guinea Worm Disease) Eradication. In: Molyneux DH, editor. Advance in Parsitology Control of Human Parasitic Diseases. London: 2007. [Google Scholar]
- 4.Cheesbrough M, Curtis S. Journal of Chemical Information and Modeling. second. Vol. 53. Cambridge: Cambrige University press; 2013. District laboratory practice in tropical countries; pp. 1689–1699. second. [Google Scholar]
- 5.Belcher DW, Wurapa FK, Ward WB, L I. Guinea worm in southern Ghana: its epidemiology and impact on agricultural productivity. Am J Trop Med Hyg. 1975;24(2):243–9. doi: 10.4269/ajtmh.1975.24.243. [DOI] [PubMed] [Google Scholar]
- 6.Ilegbodu VA, Kale OO, Wise RA, Christensen BL, Steele JH, Chambers LA. Impact of Guinea worm disease on children in Nigeria. Am J Trop Med Hyg. 1986;35(5):962–4. doi: 10.4269/ajtmh.1986.35.962. [DOI] [PubMed] [Google Scholar]
- 7.Hopkins DR, Ruiz-Tiben E, Ruebush TK, Diallo N, Agle A, Withers PC. Dracunculiasis Eradication: Delayed, not denied. Am J Trop Med Hyg. 2000;62(2):163–8. doi: 10.4269/ajtmh.2000.62.163. [DOI] [PubMed] [Google Scholar]
- 8.Savioli L, Daumerie D. Working to overcome the global impact of neglected tropical diseases: first WHO report on neglected tropical diseases. 2010:184. [Google Scholar]
- 9.Muller R. In: Zoonoses. First edit. Macpherson CNLPSC, editor. London: Unwin Hyman Ltd; 1991. pp. 204–23. [Google Scholar]
- 10.Melorose J, Perroy R, Careas S. Opportunities for control of Dracunculiasis. Statew Agric L Use Baseline. 2015:1. 2015. [Google Scholar]
- 11.WHO/CDC. WHO Collaborating Center for Research Training and Eradication of Dracunculiasis, Guinea Worm Wrap Up #236. Atlanta, USA: 2015. Sep, [Google Scholar]
- 12.WHO/CDC. WHO Collaborating Center for Research Training and Eradication of Dracunculiasis, Guinea Worm Wrap Up #234. Centers for Disease Control and Prevention; Atlanta, USA: 2015. Jun, [Google Scholar]
- 13.Watts S. Dracunculiasis in Africa in 1986: its geographic extent, incidence, and at-risk population. Am J Trop Med Hyg. 1987;37:119–25. doi: 10.4269/ajtmh.1987.37.119. [DOI] [PubMed] [Google Scholar]
- 14.WHO. Dracunculiasis eradication – global surveillance summary, 2014 Weekly Epidemiological Record. 2015 May;90(19):201–16. [PubMed] [Google Scholar]
- 15.WHO/CDC. GUINEA WORM WRAP-UP #239. Atlanta, USA: 2016. [Google Scholar]
- 16.WHO. Weekly epidemiological record. 2015 Jul;90(31):381–92. [Google Scholar]
- 17.Eyck TD. Report of an outbreak of dracunculiasis in Ethiopia. Ethiop Med J. 1971;9(3):149–52. [PubMed] [Google Scholar]
- 18.FMoH. EDEP. Who support to ethiopian dracunculiasis eradication program. Gw handover document final version. Addis Ababa, Ethiopia: 2014. [Google Scholar]
- 19.EDEP. WHO SUPPORT TO ETHIOPIAN DRACUNCULIASIS ERADICATION PROGRAM. Addis Ababa, Ethiopia: 2015. [Google Scholar]
- 20.Adugna A. Ethiopian Demography and Health. [Internet]. Available from: http://www.ethiodemographyandhealth.org/Gambella.html.
- 21.WHO. Dracunculiasis eradication – global surveillance summary. Wkly Epidemiol Rec. 2012;87(19):177–88. [PubMed] [Google Scholar]
- 22.WHO. World Health Organization. 2015 Jul 3; Online Atlas. See http://apps.who.int/dracunculiasis/dradata/
- 23.Kebede Z. Scientific Expert Group Meeting on operational research questions of programmatic importance for Dracunculiasis Eradication; 12-13 January 2015; Geneva: WHO, HQ; [Google Scholar]
- 24.Ruiz-Tiben HD, E Strategies for dracunculiasis eradication. Bull World Health Organ. 1991;69(5):533–40. [PMC free article] [PubMed] [Google Scholar]
- 25.Kappus KD, Hopkins DR, Ruiz-Tiben E, Imtiaz R, Anderson J, Azam M, Attiq A. A strategy to speed the eradication of dracunculiasis. World Health Forum. 1991;12:220–5. [PubMed] [Google Scholar]
- 26.Peries H, Carel de R, N Y. Monitoring and evaluation of Guinea Worm Eradication. Eval Program Plan. 1998;21:393–498. [Google Scholar]
- 27.Eberhard ML, Ruiz-Tiben E, Hopkins DR, Farrell C, Toe F, Craig AWP, et al. The Peculiar Epidemiology of Dracunculiasis in Chad. Am J Trop Med Hyg. 2014;90(1):61–70. doi: 10.4269/ajtmh.13-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.EDEP. Ethiopian Dracunculiasis Eradication Program (EDEP) National Review Meeting; 14-15 December 2015; Gambella. 2015. [Google Scholar]
- 29.Alew OO, Peters N. Ethiopian Dracunculiasis Eradication Program (EDEP) GOG WOREDA. Meeting, National Review; 14-15 December 2015; Gambella, Ethiopia: 2015. [Google Scholar]
- 30.Peries H, C S. Global eradication of Guinea worm. Parasitol Today. 1997;13:431–7. doi: 10.1016/s0169-4758(97)01143-5. [DOI] [PubMed] [Google Scholar]
- 31.Johnson S, J V. Dracontiasis in Rajasthan. Epidemiology of dracontiasis in Barmer District, Western Rajasthan, India. Int J Epidemiol. 1982;11:26–30. doi: 10.1093/ije/11.1.26. [DOI] [PubMed] [Google Scholar]
- 32.Bhatt AN, P KH. Guinea-worm infection in Banaskantha District of Gujarat-some important epidemiological aspects. Indian J Med Sci. 1978;32:1–4. [PubMed] [Google Scholar]
- 33.Henderson PL, Fontaine RE, K G. Guinea worm disease in northern Uganda: a major public health problem controllable through an effective water programme. Int J Epidemiol. 1988;17:434–40. doi: 10.1093/ije/17.2.434. [DOI] [PubMed] [Google Scholar]
- 34.Udonsi JK. Control of endemic dracontiasis by provision of water supply in rural communities of Imo State, Nigeria. Public Health. 1987;101:63–70. doi: 10.1016/s0033-3506(87)80030-6. [DOI] [PubMed] [Google Scholar]
- 35.Edungbola LD, Watts SJ, Alabi TO, B A. The impact of a UNICEF assisted rural water project on the prevalence of guinea worm disease in Asa, Kwara State, Nigeria. Am J Trop Med Hyg. 1988;39:79–85. doi: 10.4269/ajtmh.1988.39.79. [DOI] [PubMed] [Google Scholar]
- 36.Afele M. Countdown to wipe out guinea-worm in Ghana. Bull World Heal Organ. 2009;87:649–50. doi: 10.2471/BLT.09.010909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.A N. Towards global Guinea worm eradication in 2015: the experience of South Sudan. Int J Infect Dis. 2013:17e577–e58. doi: 10.1016/j.ijid.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Gebre T, Alamerew D, Tesfaye G. Dracunculiasis. In: Birhane Y, H D, K H, editors. Epidemiology and Ecology of Health and Disease in Ethiopia. Addis Ababa: Shama Books; 2006. pp. 674–691. [Google Scholar]
- 39.Robert B, Yang G, Knopp S, Utzinger J, T M. Surveillance and response: Tools and approaches for the elimination stage of neglected tropical diseases. Acta Trop. 2015;141:229–34. doi: 10.1016/j.actatropica.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 40.Hopkins D. Disease Eradication. N Engl J Med. 2013;368:54–63. doi: 10.1056/NEJMra1200391. [DOI] [PubMed] [Google Scholar]
- 41.WHO/CDC: Centers for Disease Control and Prevention. Progress toward global eradication of dracunculiasis, January 2005–May 2007. MMWR Morb Mortal Wkly Rep. 2007;56:813–7. [PubMed] [Google Scholar]
- 42.TCC. Innovation speeds up Guinea worm containment in South Sudan. Atlanta, USA: 2013. [accessed 22 Apri]. Available at: http://www.cartercenter.org/news/multimedia/HealthPrograms/InnovationSpeedsContainmentOf-GuineaWorm.html. [Google Scholar]
- 43.Glenshaw MT, Roy S, Ruiz-Tiben E, Downs P, W J, E M. Guinea Worm Disease Outcomes in Ghana: Determinants of Broken Worms. Am J Trop Med Hyg. 2009;81(2):305–12. [PubMed] [Google Scholar]
- 44.Lyons G. The control of guinea-worm with abate: a trial in a village of north-west Ghana. Bull WHO. 1973;49:215–6. [PMC free article] [PubMed] [Google Scholar]
- 45.Morenikeji OA, A B, O A. Evaluation of the effectiveness of Abate used for the treatment of community drinking ponds in the Nigeria Guinea worm Eradication Programme. Int J Nat Appl Sci. 2005;1(1):55–8. [Google Scholar]
- 46.Hopkins D, Richards F, Jr, Ruiz-Tiben E, Emerson P, Withers P., Jr Dracunculiasis, onchocerciasis, schistosomiasis, and trachoma. Ann N Y Acad Sci. 2008;1136:45–52. doi: 10.1196/annals.1425.015. [DOI] [PubMed] [Google Scholar]
- 47.Belcher DW, Wurapa FK, Ward WB, L I. Guinea worm in southern Ghana: its epidemiology and impact on agricultural productivity. Am J Trop Med Hyg. 1975;24(2):243–9. doi: 10.4269/ajtmh.1975.24.243. [DOI] [PubMed] [Google Scholar]
- 48.Paul John E. A field test report of implementation planning and a cost-benefit model vor Guinea worm adication in Pakistan. 1988:231. WASH F Rep No. [Google Scholar]
- 49.Hopkins D. The guinea worm eradication effort: Lessons for future. Emerg Infect Dis. 1998;4(3) doi: 10.3201/eid0403.980319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donald H. GUINEA WORM DISEASE (DracuncLuliasis) VBC Trop Dis. 1990;(4) [Google Scholar]
- 51.WHO: World Health Organization. Dracunculiasis, heightened surveillance: the key to success (updated version). DVD. Geneva, Switzerland: 2012. [Google Scholar]
- 52.Strieker G. Foul watery serpent DVD. USA: Cielo Productions; 2010. [Google Scholar]
- 53.Braide EI, Obono OMN. Sustainability in guinea worm eradication programme. 21st WEDC Conference; Kampala, Uganda. 1995. [Google Scholar]
- 54.Taheh A, Cairncross S, M G. The impact of health education to promote cloth filters on dracunculiasis prevalence, in the northern region Ghana. SocSci. 1996;43(8):1205–21. doi: 10.1016/0277-9536(95)00383-5. [DOI] [PubMed] [Google Scholar]
- 55.Cairncross S, Braide EI, B S. Community participation in the eradication of guinea worm disease. Acta Trop. 1996;61:121–36. doi: 10.1016/0001-706x(95)00106-o. [DOI] [PubMed] [Google Scholar]
- 56.Bruce A, Hennessey KA, Nevio Z, Jean-Marc O, C S. When Is a Disease Eradicable? 100 Years of Lessons Learned. Am J Public Heal. 2000;90:1515–20. doi: 10.2105/ajph.90.10.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Julius C. The last bastions of guinea-worm disease. Bull World Heal Organ. 2014;92:854–5. doi: 10.2471/BLT.14.021214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Avicenna A-1037. The Canon of Medicine. 4:138–1484. [Google Scholar]
- 59.Hopkins Donald R, Ruiz-Tiben Ernesto, Eberhard Mark L, R SL. Progress toward Global Eradication of Dracunculiasis - January 2013–June 2014. MMWR. 2014 Novemb;63(46) [PMC free article] [PubMed] [Google Scholar]
- 60.Michele B. The Tail End of Guinea Worm- Global Eradication without a Drug or a Vaccine. New Engl j med. 2007;356:25. doi: 10.1056/NEJMp078089. [DOI] [PubMed] [Google Scholar]
- 61.Klepac P, Funk S, Hollingsworth TDE, Metcalf CJ, H K. Six challenges in the eradication of infectious diseases. Epidemics. 2015;10:97–101. doi: 10.1016/j.epidem.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saint-Victor DS, Omer SB. Vaccine refusal and the endgame: Walking the last mile first. Philos Trans R Soc B. 2013;368:20120148. doi: 10.1098/rstb.2012.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O’Reilly KM, Chauvin C, Aylward RB, Maher C, Okiror S, Wolff C, et al. A statistical model of the international spread of wild poliovirus in Africa usedto predict and prevent outbreaks. PLoS Med. 2011;8:e100110. doi: 10.1371/journal.pmed.1001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Black M, United Nations International Water Decade 1981–1990. [Accessed 30 June 2015];1998 Available at http://www.un.