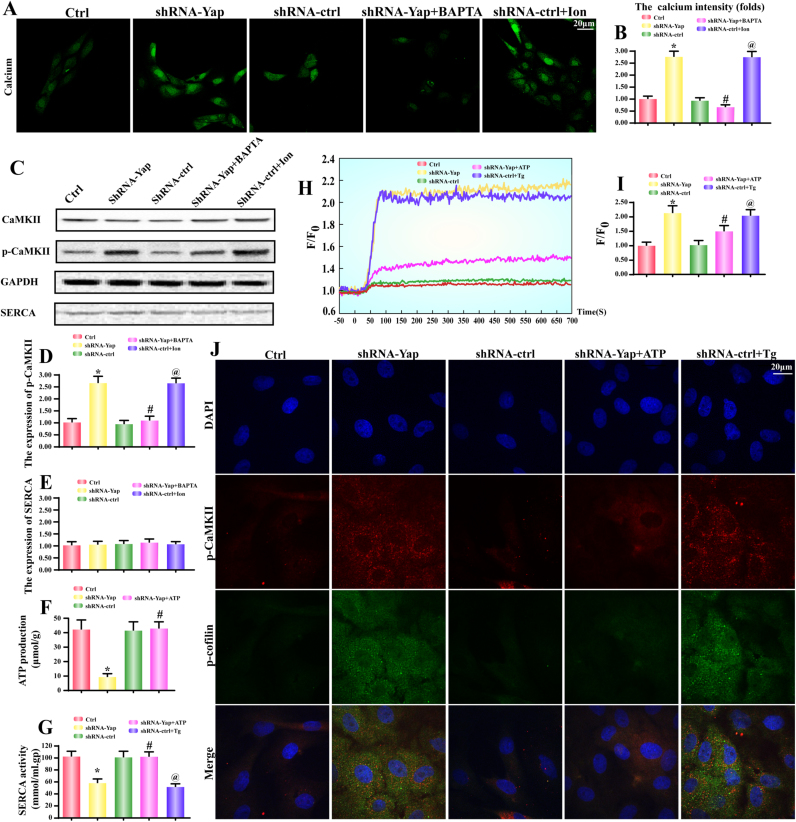
Fig. 3.
CaMKII and cofilin phosphorylation were droved by SERCA-mediated [Ca2+]i overload. A-B. The intracellular calcium ([Ca2+]i) intensity was measured by Fura-2 A.M. BAPTA, a calcium chelator, was used to reduce the level of [Ca2+]i. Ionomycin (Ion), a calcium agonist, was applied to induce [Ca2+]i overload. C-D. The CaMKII was phosphorylated by [Ca2+]i overload. C and E. The change of SERCA expression. F. The ATP production was also decreased in Yap-deleted HepG2 cells. G. The alteration of SERCA activity. SERCA activity was declined in response to Yap loss. Whereas exogenous treatment of ATP could reverse SERCA activity. H-I. The [Ca2+]i map via confocal microscopy by Fura-2 A.M. Fluorescence intensity of Fura-2AM was measured by excitation wavelengths of 340 nm and emission wavelengths of 500 nm, respectively. Data (F/F0) were obtained by dividing fluorescence intensity (F) by (F0) at resting level (t=0) which was normalized by control groups. Exogenous treatment of ATP was used to reverse the SERCA activity. Thapsigargin (Tg), an inhibitor of SERCA was used to block SERCA activity. J. The co-staining of p-CaMKII and p-cofilin. Recovery of SERCA via exogenous ATP reduced p-CaMKII and p-cofilin in Yap-deleted HepG2 cells. While inhibition of SERCA by Tg caused the increases in p-CaMKII and p-cofilin in shRNA-control HepG2 cells. *P < 0.05 vs. control group; #P < 0.05 vs. shRNA-Yap group, @ P < 0.05 vs. shRNA-ctrl group.