Figure 6.
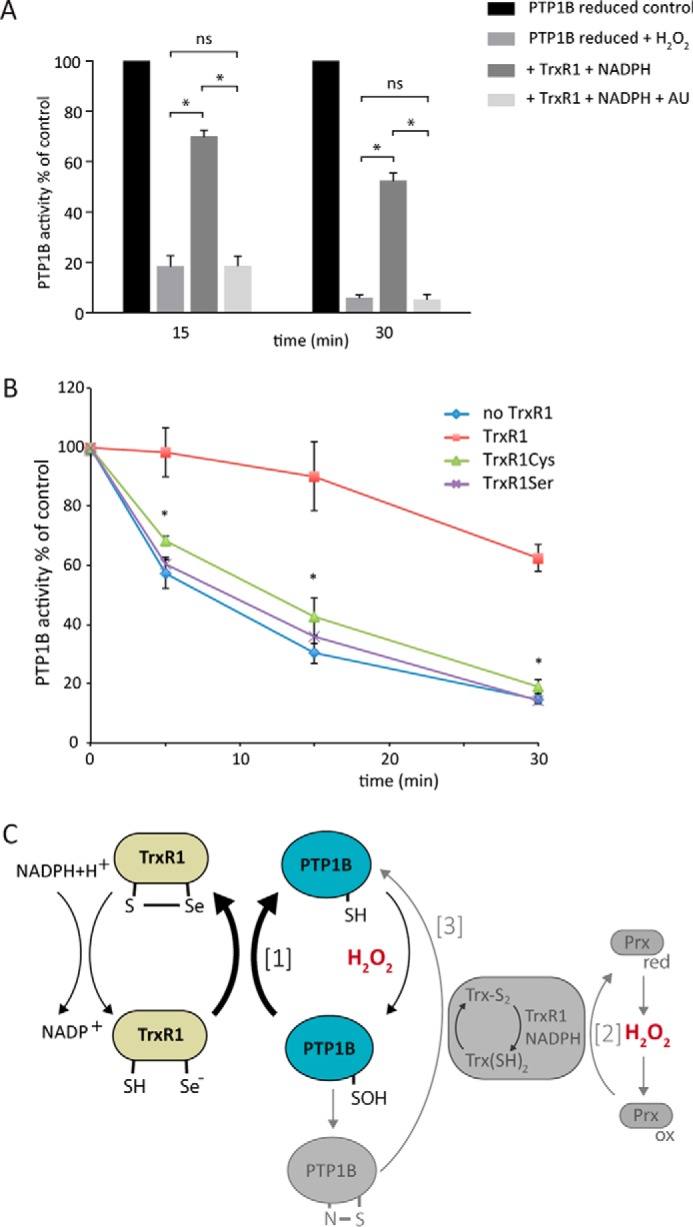
The active-site selenocysteine residue of TrxR1 is indispensable for protection of PTP1B from H2O2-mediated inactivation. A, inhibition of TrxR1 protection by auranofin. Reduced PTP1B (600 nm) was treated with or without TrxR1 (0.5 μm, 9.75 units/mg), NADPH (200 μm), and auranofin (AU, 1 μm). PTP activity was measured at the indicated times after addition of 100 μm H2O2 (n = 3; mean ± S.E.; *, p < 0.05). B, lack of protection by active-site mutants of TrxR1. Reduced PTP1B was exposed to 100 μm H2O2 in the presence of wild-type TrxR1 (18 units/mg) or variants where the active-site SelCys was mutated to Cys (TrxR1Cys) or Ser (TrxR1Ser) and then assayed for PTP activity as in A (n = 3; mean ± S.E.; *, p < 0.05). C, schematic of how the redox activity of the Trx/TrxR/Prx system could regulate PTP1B activity. The bold arrows show the reaction of TrxR1 (green) with the sulfenic acid of PTP1B that, we propose, explains protection of PTP1B activity (blue) by TrxR1 as observed in this study (reaction 1). Additional activities of the Trx system in relation to PTP regulation, as described previously in the literature, are schematically shown in gray. The full scheme is discussed in the text.
