Figure 5.
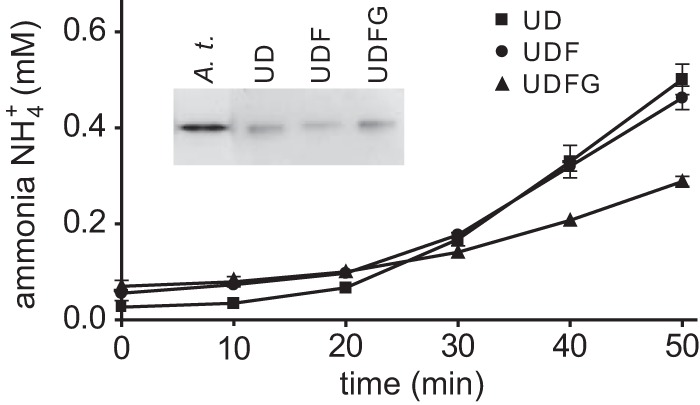
In vitro activation of different urease–UAP complexes. Urease–UreDS (UD)- and urease–UreDSUreF (UDF)- and urease–UreDS–UreF–UreG (UDFG) complexes, transiently expressed in N. benthamiana and purified using N-terminally Strep-tagged UreD, were incubated with 5 μm NiCl2, 2 mm NaHCO3, and 5 mm urea. Ammonia release by urease was measured and normalized to the relative amount of purified urease protein present in each assay, which was quantified by Western blot. The experiment was repeated three times (n = 3, error bars = S.D.). After 16 h incubation, the samples and a clarified extract of Arabidopsis wild-type leaves (A.t.) as positive control were separated by native gel electrophoresis and tested for in-gel urease activity (inset).
