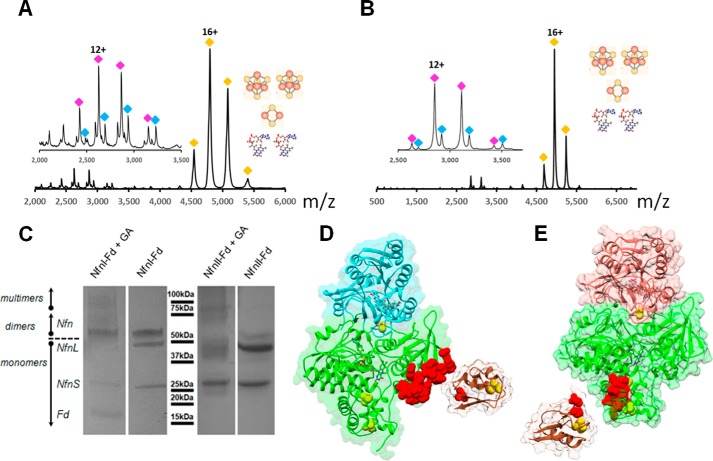
Figure 4.
Native and chemical cross-linking mass spectrometry of NfnI and NfnII. Native mass spectrum of NfnI complex (A) and NfnII complex (B) in the gas phase. Yellow diamonds, charge states of the intact complex centered around charge 16+. After deconvolution, the intact NfnI complex is 86,407 Da (expected: 86,425 Da) and NfnII is 88,951 Da (expected 88,901 Da), consistent with complexes containing one small and large subunit, two FAD molecules, two [4Fe–4S] clusters, and one [2Fe–2S] cluster. Under conditions of low collision energy (80 V for NfnI and 60 V for NfnII), the subunits dissociated (insets in A and B). Blue and magenta diamonds, charge envelopes of small subunits with and without one FAD cofactor. C, SDS-PAGE of glutaraldehyde (GA)–cross-linked NfnI–Fd and NfnII–Fd complexes. LC-MS analysis revealed that ferredoxin (brown) interacts with the large subunits (green) of NfnI (D) and NfnII (E). Red space-filling regions show cross-linked peptides of NfnI and NfnII. Small subunits are colored in cyan and red, respectively. In all experiments, Nfn enzymes were used “as purified.” The NfnI structure is PDB entry 5JFC; NfnII structure is PDB entry 5VJ7.