Figure 1.
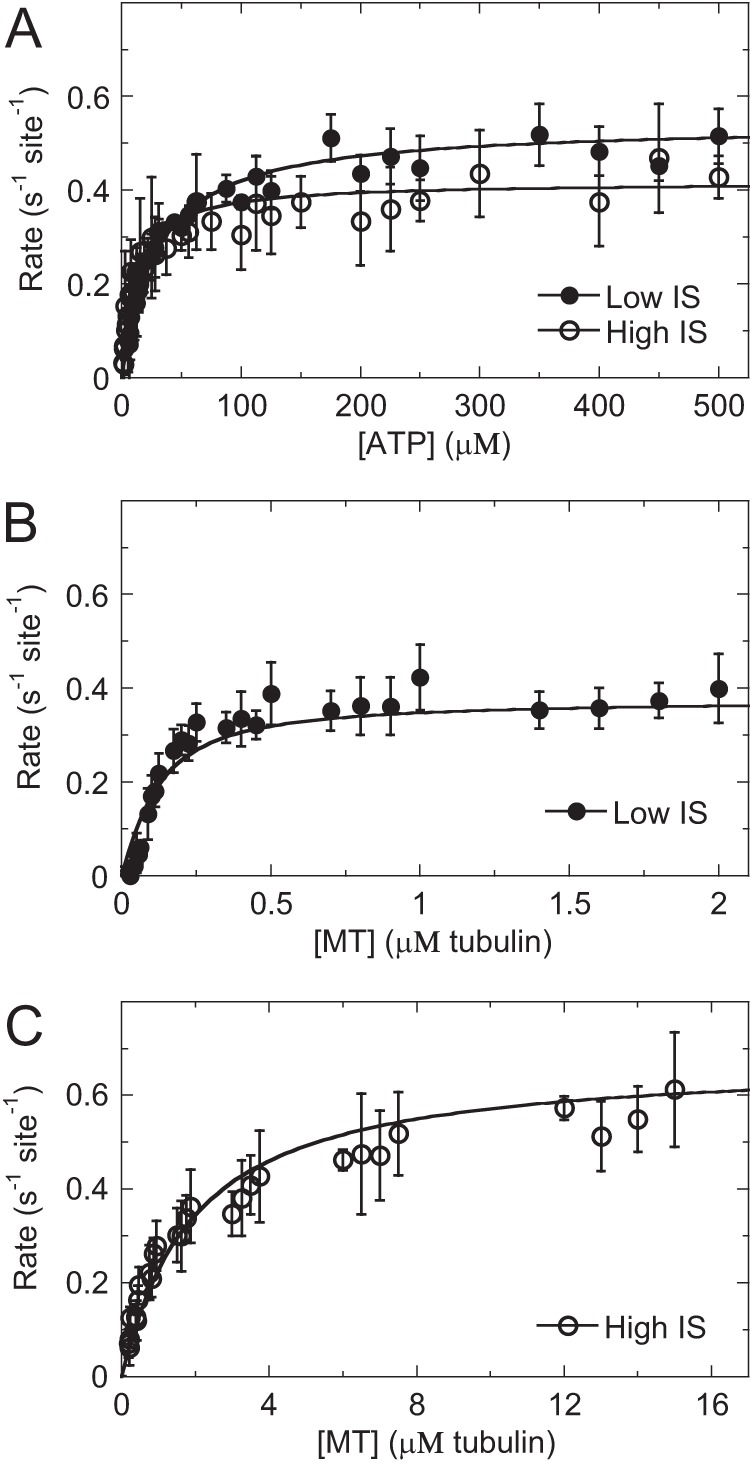
Steady-state kinetics of Cin8 motors reveals typical microtubule-stimulated ATPase. A, ATP-stimulated activity for Cin8 at low (closed circles) and high (open circles) ionic strength (IS). Final concentrations are 0.1 μm Cin8-His, 2 μm microtubules, and 5–1000 μm ATP. Data were fit to Equation 1. Low, kcat = 0.54 ± 0.01 s−1 site−1 and Km, ATP = 29 ± 2 μm. High, kcat = 0.42 ± 0.01 s−1 site−1 and Km, ATP = 12 ± 1 μm. B, microtubule-stimulated ATPase activity for Cin8 at low ionic strength. Final concentrations are 0.01 μm Cin8-His, 0–2 μm microtubules, and 1 mm ATP. Data were fit to Equation 2 to yield kcat at 0.38 ± 0.01 s−1 site−1 and K0.5, MT = 0.08 ± 0.02 μm. C, microtubule-stimulated activity for Cin8 at high ionic strength. Final concentrations are 0.2 μm Cin8, 0–30 μm microtubules, and 1 mm ATP. Data were fit to Equation 2 to yield kcat at 0.68 ± 0.02 s−1 site−1 and K0.5, MT = 1.8 ± 0.2 μm. kbasal was 0.01 ± 0.02 s−1 site−1 at low and 0.02 ± 0.02 s−1 site−1 at high ionic strength.
