Abstract
Objective
To assess physicians’: 1) knowledge and attitudes about hepatitis A disease and hepatitis A (HepA) vaccine, 2) child care and school HepA vaccine mandates, 3) practices related to HepA vaccine delivery, 4) factors associated with strongly recommending HepA vaccine to all 1- to 2-year-olds, and 5) feasibility of implementing HepA catch-up vaccination at health maintenance visits.
Methods
A national survey was conducted among representative networks of pediatricians and family medicine physicians (FMs) from March to June, 2014 via e-mail or mail on the basis of provider preference.
Results
Response rates were 81% (356 of 440) among pediatricians and 75% (348 of 464) among FMs. Less than 50% correctly identified that hepatitis Avirus (HAV) infection is usually asymptomatic in young children and that morbidity from HAV disease increases with age. Ninety-two percent of pediatricians and 59% of FMs strongly recommend HepA vaccine for all 1- to 2-year-olds. In addition to practice specialty, belief that HepA vaccine is required for kindergarten enrollment was the most robust predictor of strong physician recommendation.
Conclusions
Gaps in knowledge regarding HAV infection and hepatitis A recommendations and lack of a strong recommendation for routine HepA vaccination of young children among FMs likely contribute to suboptimal coverage. Closing knowledge gaps and addressing barriers that prevent all physicians from strongly recommending HepA vaccine to 1- to 2-year-olds could help increase HepA vaccine coverage and ultimately improve population protection against HAV infection.
Keywords: hepatitis A, hepatitis Avaccine, catch-up vaccination, pediatricians, family medicine physicians
Hepatitis a is a vaccine-preventable communicable disease of the liver caused by the hepatitis A virus (HAV). Whereas children younger than 5 years of age with HAV infections are usually asymptomatic, morbidity and mortality increase in older age groups. Serious complications due to HAV infection are rare but can result in liver failure and death.1 Rates of HAV disease have decreased overall since vaccine introduction, however, since 2007 rates have been higher among adults than among children age 0 to 9 years.1,2 In recent years the average age of HAV-related hospitalizations and deaths has increased, and persons hospitalized for hepatitis A are more likely to have liver diseases and other comorbid medical conditions.3,4
The HepA vaccine was recommended incrementally by the Advisory Committee on Immunization Practices (ACIP) starting in 1996.5,6 In 2006, the ACIP recommended routine HepA vaccination for all children aged 12 to 23 months, vaccination for persons who are at increased risk for infection, or for any person wishing to obtain immunity.7 Children who are not vaccinated by age 2 years can be vaccinated at subsequent visits, and catch-up vaccination of unvaccinated children aged 2 through 18 years can be considered, on the basis of individual clinical decision-making.
Despite the demonstrated safety and efficacy of HepA vaccine,7 2-dose coverage for HepA vaccine is poor, especially among adults. In 2015, for children aged 19 to 35 months, vaccine coverage was 85.8% and 59.6%, for ≥ 1 and ≥ 2 doses, respectively,8 and, in 2012, coverage for children aged 13 to 17 years was approximately 60% and 48% for 1 and ≥ 2 doses respectively, on the basis of preliminary data.9 Although protection from HAV has increased among children because of the success of childhood vaccination, the antibody to HAV (anti-HAV) seroprevalence has decreased significantly among adults 20 years of age and older because of less exposure to infected children and continued low vaccination coverage.10 This is resulting in increased hepatitis A susceptibility among adults.2,10
High vaccine coverage rates among children as a result of routine childhood vaccination programs has been shown to provide population protection from HAV over time.11 Therefore, it is important to maximize HepA vaccine coverage for children and adolescents aged 2 to 18 years to improve future vaccination rates and protection of adults. Among adults aged 19 years and older surveyed in 2014 the total ≥ 2 dose coverage was 9%; and among high risk adults aged 19 years or older for whom the HepA vaccine is recommended, coverage was 16% among travelers and 13.8% among persons with chronic liver conditions.12
To inform these efforts, we conducted a national survey of pediatricians and family medicine physicians (FMs) to examine their current HepA vaccination practices and the feasibility of implementing adolescent catch-up vaccination. Our objectives were to describe: 1) knowledge and attitudes about HAV infection and HepA vaccine, 2) knowledge and attitudes regarding child care and school HepA vaccine mandates, 3) practices related to HepA vaccine delivery, 4) factors associated with strongly recommending HepA vaccine to all children aged 1 to 2 years, and 5) feasibility of implementing HepA catch-up vaccination at health maintenance visits.
Methods
Study Setting
A survey was administered via Internet or postal mail from March through June of 2014 to primary care physicians recruited from the American Academy of Pediatrics (AAP) and American Academy of Family Physicians (AAFP). The human subjects review board at the University of Colorado approved this study as exempt research, not requiring written informed consent.
Study Population
A method was developed for obtaining rapid and high response rates to surveys about policy-relevant immunization issues as part of a US Centers for Disease Control and Prevention-funded project.13 Networks of physicians were recruited from the AAPand the AAFP, whoagreed to respond to several surveys each year.14 A population-based sampling matrix was constructed using demographic and practice data from randomly drawn samples of the AAP and AAFP memberships. Using population-based estimates, matrix quotas were created, which crossed US regions, practice location, and type of practice. Cells were then filled by randomly selecting fromall of therecruits to yield a total ofapproximately 400 physicians in each network. In a previous study, demographic characteristics, practice attributes, and reported attitudes about a range of vaccination issues were generally similar when network physicians were compared with physicians of the same specialty randomly sampled from the American Medical Association master physician listing.14
Survey Design
The survey instrument was developed collaboratively with the US Centers for Disease Control and Prevention, incorporating the format of previously administered surveys with revised content and pretested in community advisory panels consisting of pediatricians and FMs from across the country. The survey was then pilot-tested among 72 pediatricians and 20 FMs. In addition to questions about physician and practice characteristics, the survey assessed knowledge and attitudes about HAV infection and HepA vaccine, knowledge and attitudes regarding child care and school HepA vaccination mandates, current practices related to HepA vaccine delivery, factors associated with strongly recommending HepA vaccine to all children aged 1 to 2 years, and the feasibility of implementing a HepA catch-up vaccination at health maintenance visits.
Responses to knowledge questions were either agree or disagree whereas responses to attitudes, current practices, and barriers questions used 4-point Likert scales.
Survey Administration
The survey was administered using a Web-based Internet program or postal mail on the basis of each physician’s preference. The Internet group received an initial email with a link to the survey and up to 8 e-mail reminders to complete the survey, whereas the mail group received an initial mailing and up to 2 additional mailed surveys at 2-week intervals. The Internet nonresponders also received up to 2 paper surveys via mail.
Analytic Methods
Internet and mail surveys were pooled, because provider attitudes have been reported to be comparable when obtained by either method.14 Chi-square tests were used to compare the proportions of pediatricians and FMs who correctly reported information about HAV infection and vaccine. We compared respondents with nonrespondents using a t test, chi-square, and Mantel-Haenszel chi-square tests, as appropriate. We compared pediatrician and FM responses using chi-square and Mantel-Haenszel chi-square tests. We conducted a multivariable analysis with the dependent variable of strongly recommending the HepA vaccine according to current ACIP guidelines. Independent variables included physician specialty, practice setting, practice region, having managed patient(s) with HAV infection, attitudes about HepA vaccine, belief in state requirements for HepA vaccination, and actual state requirements for HepA vaccination. These independent variables were chosen on the basis of a review of the literature and our a priori hypotheses regarding factors most likely to affect physicians’ strength of recommendation for HepA vaccine. The most up-to-date and available state requirements for HepA vaccination were obtained from Immunization Action Coalition data.15 Independent variables with a P value of .25 or less were included in the multivariable logistic regression model. Variables were retained in the final model for P < .05. Analyses were performed using SAS software, version 9.4 (SAS Institute Inc, Cary, NC).
Results
Response Rates and Sample Characteristics
Response rates were 81% (356 of 440) among pediatricians and 75% (348 of 464) among FMs. Characteristics of respondents compared with nonrespondents, as well as additional characteristics of the study population are shown in Table 1. FM respondents were more likely to be female than nonrespondents with additional minor differences in distribution according to region.
Table 1.
Comparison of Respondents and Nonrespondents and Additional Characteristics of Respondents’ Practices
| Respondents, %
|
Nonrespondents, %
|
|||
|---|---|---|---|---|
| Physician and Practice Characteristics | Peds (n = 356) | FM (n = 348) | Peds (n = 84) | FM (n = 116) |
| Female sex | 65 | 48* | 62 | 32* |
| Setting | ||||
| Private practice | 76 | 66 | 81 | 73 |
| Hospital or clinic | 22 | 23 | 17 | 19 |
| HMO | 2 | 10 | 2 | 8 |
| Location | ||||
| Urban, inner city | 14 | 39 | 17 | 30 |
| Urban, not inner city | 75 | 53 | 77 | 59 |
| Rural | 11 | 8 | 6 | 11 |
| Region | ||||
| Midwest | 21 | 30* | 14 | 25* |
| Northeast | 22 | 14 | 24 | 13 |
| South | 33 | 33 | 42 | 45 |
| West | 24 | 23 | 20 | 17 |
| Mean age in years (SD) | 50.4 (10.4) | 53.4 (7.9) | 50.2 (11.6) | 53.6 (8.0) |
| Proportion of patients age 0 to 10 years | ||||
| < 10% | 2 | 47 | ||
| 10%–29% | 1 | 24 | ||
| 30%–49% | 16 | 13 | ||
| ≥ 50% | 81 | 15 | ||
| Proportion of patients age 11 to 18 years | ||||
| < 10% | 1 | 37 | ||
| 10%–29% | 21 | 32 | ||
| 30%–49% | 70 | 17 | ||
| ≥ 50% | 7 | 15 | ||
| Proportion of patients with Medicaid or CHIP | ||||
| < 10% | 23 | 36 | ||
| 10%–24% | 19 | 26 | ||
| 25%–49% | 22 | 19 | ||
| ≥ 50% | 37 | 19 | ||
| Proportion of Hispanic/Latino patients | ||||
| < 10% | 43 | 62 | ||
| 10%–24% | 30 | 22 | ||
| ≥ 25% | 27 | 16 | ||
| Proportion of black/African American patients | ||||
| < 10% | 43 | 66 | ||
| 10%–24% | 35 | 23 | ||
| ≥ 25% | 22 | 11 | ||
CHIP indicates Children’s Health Insurance Program; FM, family physicians; HMO, health maintenance organization; and Peds, pediatricians.
P ≤ .05 for comparison between responders and nonresponders.
Knowledge and Attitudes about HepA Vaccine
Among respondents, 40% of pediatricians compared with 60% of FMs reported previously managing a patient with HAV infection in their clinical practice (P < .0001) and 67% of pediatricians compared with 60% of FMs were aware of hepatitis A outbreaks that had occurred in their state (P = not significant). Table 2 shows knowledge of facts about HAV infection and HepA vaccine. In general, knowledge was either similar between specialties or higher among pediatricians. Substantial percentages of physicians from both specialties responded that they did not know or were unsure about a number of statements, with FMs being more likely to report they were unsure about statements regarding children. Attitudes about HAV infection and the vaccine are shown in Figure 1. The pattern of responses was similar between specialties, although pediatricians were more likely to disagree that HAV infection was usually severe among children and to agree that it was usually severe among adults. Pediatricians were also significantly more likely to strongly agree that the vaccine was beneficial for all age groups and safe and cost effective from a societal perspective.
Table 2.
Physicians’ Knowledge About HAV Infection and HepA Vaccine
| Survey Questions | Peds, % (n = 356) | FM, % (n = 344) |
|---|---|---|
| In children younger than 6 years, HAV infection usually causes symptoms such as nausea, abdominal pain, and jaundice* | ||
| Agree | 62 | 50 |
| Disagree† | 30 | 27 |
| Don’t know/not sure | 8 | 23 |
| Children younger than 6 years old are often the source of HAV infection for adults* | ||
| Agree† | 65 | 52 |
| Disagree | 13 | 20 |
| Don’t know/not sure | 21 | 28 |
| HAV is a common vaccine-preventable disease acquired during travel | ||
| Agree† | 94 | 94 |
| Disagree | 2 | 3 |
| Don’t know/not sure | 4 | 3 |
| In the United States, most HAV infections result from food-borne outbreaks | ||
| Agree† | 85 | 85 |
| Disagree | 9 | 8 |
| Don’t know/not sure | 6 | 8 |
| Approximately 50% of persons with HAV infection do not have a source identified for their infection | ||
| Agree† | 76 | 78 |
| Disagree | 4 | 5 |
| Don’t know/not sure | 20 | 17 |
| Long-term studies indicate that protective levels of anti-HAV antibodies persist 10 years or more after HepA vaccination* | ||
| Agree† | 75 | 65 |
| Disagree | 1 | 2 |
| Don’t know/not sure | 24 | 33 |
| The safety of the HepA vaccine has been monitored for almost 20 years through post-licensure studies and the vaccine adverse event reporting system* | ||
| Agree† | 92 | 84 |
| Disagree | 0 | 1 |
| Don’t know/not sure | 8 | 16 |
| A large proportion of young and middle-age adults do not have protection against HAV infection | ||
| Agree† | 86 | 89 |
| Disagree | 3 | 3 |
| Don’t know/not sure | 12 | 9 |
| Mortality due to HAV infection is lower among persons aged 45 years or older compared with younger age groups | ||
| Agree | 12 | 17 |
| Disagree† | 44 | 41 |
| Don’t know/not sure | 44 | 42 |
| Morbidity due to HAV infection decreases with age | ||
| Agree | 18 | 21 |
| Disagree† | 48 | 45 |
| Don’t know/not sure | 34 | 34 |
| People with existing liver disease are at risk for getting severe liver problems if they become infected with HAV | ||
| Agree† | 90 | 92 |
| Disagree | 3 | 5 |
| Don’t know/not sure | 7 | 4 |
| Adults infected with HAV rarely miss work because of their infection | ||
| Agree | 5 | 6 |
| Disagree† | 78 | 83 |
| Don’t know/not sure | 18 | 11 |
FM indicates family medicine physicians; HAV, hepatitis A virus; HepA, hepatitis A; and Peds, pediatricians.
P < .05 using chi-square test for comparison between specialties.
Correct answer.
Figure 1.
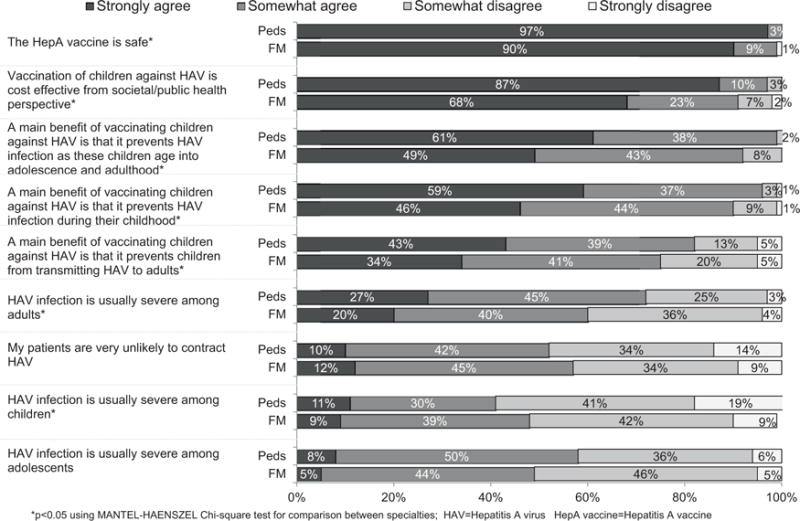
Physician knowledge and attitudes about hepatitis A virus (HAV) infection and hepatitis A (HepA) vaccine (pediatricians [Peds] n = 356; family medicine physicians [FM] n = 344). *P < .05 using Mantel-Haenszel chi-square test for comparison between specialties.
Knowledge and Attitudes Regarding Child Care and School HepA Vaccine Mandates
HepA vaccine is currently required for child care enrollment in 20 states and is required for kindergarten enrollment in 13 states.15 Only 50% (95 of 191) of physicians from states with a child care requirement for HepA vaccination knew about the requirement (pediatrician 64%, FMs 35%; P < .0001), and 66% (83 of 128) of physicians from states with a kindergarten requirement for HepA vaccination knew about this requirement (pediatricians 74%, FMs 58%; P = .05). Among those who did not think their state had a requirement, 87% (204 of 234) of pediatricians and 70% (190 of 272) of FMs reported being in support of child care requirements and 87% (193 of 223) of pediatricians and 68% (168 of 247) of FMs supported kindergarten requirements for HepA vaccine.
Practices Related to HepA Vaccine Delivery
All pediatricians and 85% of FMs reported currently administering HepA vaccine. Age when first dose was usually administered varied according to specialty. Among pediatricians compared with FMs, respectively, 75% versus 74% reported 12 to 14 months; 23% versus 10% reported 15 to 23 months; and 3% versus 16% reported 2 years or older (P < .0001). The strength of recommendation for HepA vaccine among all physicians, whether they administered the vaccine or not, according to different age groups and risk categories is shown in Figure 2. Pediatricians more strongly recommended the vaccine for all age and risk groups. Ninety-two percent of pediatricians, but only 59% of FMs strongly recommend HepA vaccine for all children aged 12 to 23 months (P < .0001).
Figure 2.
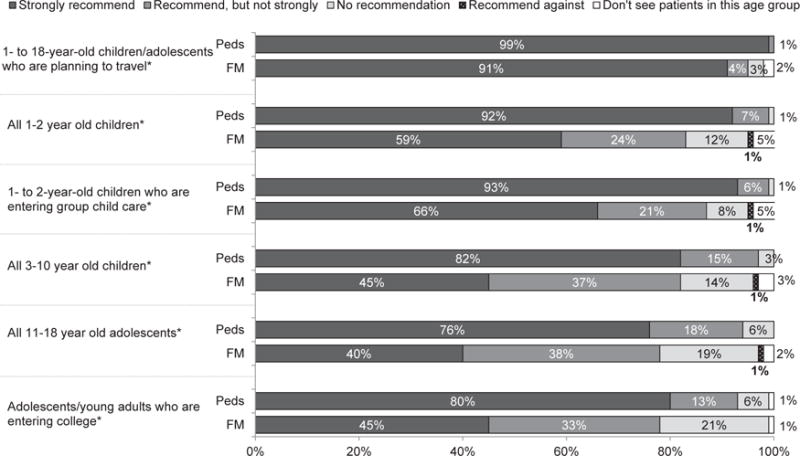
Strength of recommendation for hepatitis A (HepA) vaccine (pediatricians [Peds] n = 356, family medicine physicians [FM] n = 344). *P < .05 using chi-square test for comparison between specialties.
Specific strategies used to increase HepA vaccination rates in the practice included databases to assess whether a vaccine is needed (pediatricians 61%, FM 55%; P = .09), provider alerts (pediatricians 51%, FM 44%; P = .09), reminder/recall notices (pediatricians 27%, FM 22%; P = .15), and standing orders (pediatricians 22%, FM 24%; P = .71).
Factors Associated With Strongly Recommending HepA Vaccine to All 1- to 2-Year-Olds
Bivariate and multivariable analyses examining predictors of reporting a strong recommendation for all 1- to 2-year-old children are shown in Table 3. The strongest associations in multivariable analyses were specialty (pediatricians compared with FMs) and belief that the state in which they practiced requires HepA vaccination for kindergarten enrollment. Other significant associations included strongly agreeing that HAV infection is usually severe among adults, and having higher proportions of Medicaid, State Children’s Health Insurance Program (SCHIP), and Latino/Hispanic patients in the practice.
Table 3.
Characteristics and Attitudes Associated With a Strong Hepatitis A Recommendation, Peds and FM Combined
| Variable | Strongly Recommend for All 1- to 2-Year-Old Children
|
Bivariable Odds Ratio (95% CI) | Multivariable Adjusted Odds Ratio (95% CI) | ||
|---|---|---|---|---|---|
| Yes (n = 515), % | No (n = 149), % | P | |||
| Practice specialty | <.0001 | ||||
| Pediatrics | 62 | 19 | 6.9 (4.4–10.7) | 5.4 (3.3–8.8) | |
| Family medicine | 38 | 81 | Referent | Referent | |
| Practice setting | .002 | ||||
| Private practice | 70 | 77 | Referent | ||
| Hospital or clinic | 25 | 13 | 2.1 (1.3–3.6) | ||
| HMO | 5 | 10 | 0.6 (0.3–1.1) | ||
| Practice region | .12 | ||||
| Midwest | 26 | 26 | 0.7 (0.4–1.2) | ||
| Northeast | 17 | 24 | 0.5 (0.3–0.9) | ||
| South | 30 | 31 | 0.7 (0.4–1.2) | ||
| West | 26 | 19 | Referent | ||
| Have managed patients with HAV infection in my clinical practice | .12 | ||||
| Yes | 49 | 56 | 0.8 (0.5–1.1) | ||
| No | 51 | 44 | Referent | ||
| HAV infection is usually severe among children | .07 | ||||
| Strongly agree | 12 | 6 | 2.0 (0.9–4.1) | ||
| All other responses | 88 | 94 | Referent | ||
| HAV infection is usually severe among adolescents | .01 | ||||
| Strongly agree | 8 | 2 | 3.9 (1.2–2.9) | ||
| All other responses | 92 | 98 | Referent | ||
| HAV infection is usually severe among adults | <.0001 | ||||
| Strongly agree | 28 | 10 | 3.3 (1.9–5.8) | 3.1 (1.6–6.2) | |
| All other responses | 72 | 90 | Referent | Referent | |
| A main benefit of vaccinating children against HAV is that it prevents children from transmitting HAV to adults | .01 | ||||
| Strongly agree | 41 | 30 | 1.7 (1.1–2.4) | ||
| All other responses | 59 | 70 | Referent | ||
| A main benefit of vaccinating children against HAV is that it prevents HAV infection as these children age into adolescence and adulthood | .01 | ||||
| Strongly agree | 58 | 46 | 1.6 (1.1–2.4) | ||
| All other responses | 42 | 54 | Referent | ||
| The HepA vaccine is safe | .01 | ||||
| Strongly agree | 96 | 87 | 1.6 (1.1–2.4) | ||
| All other responses | 4 | 54 | Referent | ||
| Respondent thinks their state requires HepA vaccination for licensed child care enrollment among children age 1 year and older | <.0001 | ||||
| Yes | 29 | 8 | 5.0 (2.6–9.5) | ||
| No/don’t know | 71 | 92 | Referent | ||
| Respondents’ state actually requires HepA vaccination for licensed child care enrollment among children age 1 year and older | .004 | ||||
| Yes | 30 | 18 | 1.9 (1.2–3.1) | ||
| No | 70 | 82 | Referent | ||
| Respondent thinks their state requires HepA vaccination for kindergarten enrollment | <.0001 | ||||
| Yes | 35 | 9 | 5.5 (3.0–10.0) | 5.8 (3.0–11.3) | |
| No/don’t Know | 65 | 91 | Referent | Referent | |
| Respondents’ state actually requires HepA vaccination for kindergarten enrollment | 0.005 | ||||
| Yes | 80 | 90 | 2.2 (1.3–4.0) | ||
| No | 20 | 10 | Referent | ||
| Percent of patients with Medicaid or SCHIP | <.0001 | ||||
| 0 to 24% | 45 | 71 | Referent | Referent | |
| 25% or more | 55 | 29 | 2.9 (2.0–4.4) | 2.2 (1.3–3.5) | |
| Percent Hispanic patients | <.0001 | ||||
| 0 to 24% | 74 | 92 | Referent | Referent | |
| 25% or more | 26 | 8 | 3.84 (2.1–7.2) | 2.6 (1.3–5.4) | |
CI indicates confidence interval; FM, family medicine physicians; HAV, hepatitis A virus; HepA, hepatitis A; HMO, health maintenance organization; Peds, pediatricians; and SCHIP, State Children’s Health Insurance Program.
Independent variables with a significance level of P ≤ .25 were included in the multivariable model; adjusted odds ratios are shown only for variables that were significant at P < .05 in the final multivariable model.
Feasibility of Implementing HepA Catch-Up Vaccination
If ACIP made a recommendation for catch-up HepA vaccination at health maintenance visits for all children 2 to 18 years of age, 96% of pediatricians and 79% of FMs reported it would be very feasible to routinely assess HepA vaccination status and vaccinate children/adolescents who were not fully vaccinated; an additional 4% and 19%, respectively, indicated it would be moderately feasible. The most common barriers (pediatricians and FMs combined) to implementing a HepA vaccine catchup recommendation included infrequent visits by adolescent patients (65%); parents not thinking HAV disease was serious (39%); HepA vaccination not being required for child care or school entry (39%); difficulty obtaining immunization records to determine a patient’s HepA vaccination status (35%); and parental concerns about giving too many vaccines at 1 visit (33%).
Discussion
This study described provider knowledge, attitudes, and practices related to HAV disease and HepA vaccine. Our data demonstrate knowledge deficits related to HAV infection among physicians serving children; notably, less than half of the respondents correctly identified that HAV infection is usually asymptomatic in young children and that morbidity and mortality from HAV increase with age. Belief that HepA vaccination was required for kindergarten enrollment in the respondent’s state was the most robust predictor of a strong provider recommendation for HepA vaccination of 1- to 2-year-old patients. Variability in provider knowledge and attitudes related to hepatitis A might partially account for the low rates of HepA vaccination in young children.
In our study, FMs were less likely than pediatricians to know that childhood HepA vaccination provides long-term disease protection or that HepA vaccine safety has been monitored for nearly 2 decades. This might be because pediatricians vaccinate newborns and infants more often than FMs when safety concerns are even greater and therefore pediatricians have more awareness of adverse events reporting. They were also less likely to know that children younger than age 6 years with HAV infection are usually asymptomatic and often infect adults. A much greater proportion of FMs than pediatricians reported ever managing a patient with HAV infection (60% vs 40%). It might be that FMs have more experience with adult HAV infections, decreasing their perceived importance of childhood HepA vaccination; respondents were not asked the age of the HAV-infected patients that they had treated.
Differences in hepatitis A-related attitudes and practices according to provider specialty were also found. Less than two-thirds of FMs strongly recommended vaccination for all children aged 1 to 2 years in accordance with ACIP recommendations. FMs were significantly less likely than pediatricians to strongly agree that HepA vaccine is safe, that HepA vaccination of children is cost-effective from a public health perspective, or that childhood HepA vaccination is beneficial in protecting children from infection or reducing prevalence of HAV infection in adolescents and adults. FMs were also less likely than pediatricians to report stocking and administering HepA vaccine in their practices, and more likely to report administering the first dose of HepA vaccine after age 2. Most notably, FMs were significantly less likely than pediatricians to strongly recommend HepA vaccine for children of any age. Only 59% of FMs reported strongly recommending HepA vaccine for 1- to 2-year-old children, compared with 92% of pediatricians. This difference is concerning because the HepA vaccine has been universally recommended for this age group since 2006.16 Whereas most early childhood vaccines are administered by pediatricians,17 FMs are more likely to serve children living in rural areas,18 potentially creating disparities in HepA vaccination coverage of young children living in these areas. Provider recommendations for vaccination are one of the strongest predictors of whether children are vaccinated16; all providers who treat children should strongly recommend HepA vaccination for 1- to 2-year-olds who have not completed a 2-dose series.
The strongest association with strongly recommending HepA vaccine at the recommended age of 1 to 2 years in multivariable analysis was the respondent’s belief that his or her state requires HepA vaccination for kindergarten enrollment. Belief in a HepA vaccination requirement for childcare enrollment, as well as the actual presence of HepA vaccination requirements in the state, were not significant in adjusted analyses. Vaccination requirements for child care and school entry have been associated with increases in coverage for required vaccines19–21; therefore, it is reasonable to think that physicians who are aware of such requirements recommend vaccination more strongly for their eligible patients. Our findings that approximately half of physicians did not know their state had a child care requirement and one-third did not know their state had a kindergarten requirement suggest that one strategy to increase HepA vaccination of young children is to increase physicians’ awareness of existing vaccination requirements for school entry. For example, public health officials in jurisdictions with HepA vaccination requirements for childcare or school entry could work with state chapters of physician organizations to ensure physicians are aware of these requirements. Similarly, among physicians who did not think their state had a requirement, most were supportive of requirements, suggesting that physicians could work with public health officials, legislators, and schools or child care programs to institute requirements for HepA vaccination in states without them.
Almost all physicians surveyed agreed that implementation of HepA vaccination catch-up at well visits for unvaccinated children 2 to 18 years of age is feasible, particularly among pediatricians. Infrequent office visits by adolescent patients was the most commonly identified barrier to catch-up HepA vaccination, although physicians also reported concerns about lack of school entry requirements, parental perceptions of disease severity, and difficulty determining HepA vaccination status. Despite physician reports, incorporating HepA catch-up vaccination might be challenging because of incomplete implementation of the current ACIP recommendations, particularly by FMs. Further research is needed to determine the reasons that many FMs do not strongly recommend HepA vaccination for 12- to 23-month-old patients and to identify interventions to mitigate this barrier. Recent data indicate that about 40% of children aged 19 to 35 months are not fully vaccinated against HAV.8 While catch-up vaccination recommendations could be beneficial for these children, a better understanding of physician and patient-related factors contributing to this suboptimal coverage would help ensure successful implementation of catch-up vaccination. Of course, more complete implementation of existing ACIP HepA vaccination recommendations would reduce the need for catch-up vaccination.
Recently, the New York City Bureau of Immunization recommended HepA vaccine for all children aged 1 to 18 years who do not have 2 valid doses recorded in the Citywide Immunization Registry.22 Local recommendations like this one might help to increase HepA vaccine coverage among older children. In addition, jurisdictions with HepA vaccination requirements for childcare or school entry could work with state chapters of physician organizations to ensure physicians are aware of these requirements.
The study has 3 major limitations. Although the sample of sentinel physicians surveyed was designed to be representative of AAP and AAFP memberships, the attitudes, experiences, and practices of sentinel physicians might not be fully generalizable. However, responses from the sentinel networks surveyed have been shown to be comparable with those from randomly sampled physicians.16 Additionally, although this survey had a high response rate, nonrespondents might differ from respondents in ways that could not be measured. Finally, physicians’ self-reported knowledge and behaviors regarding hepatitis A and HepA vaccine might not accurately reflect clinical practice.
Conclusions
Our findings show lack of knowledge among physicians on some aspects of HAV infection and HepA vaccination, and notable differences in HepA vaccination attitudes and behaviors according to physician specialty. These knowledge and practice deficits might contribute to the current suboptimal coverage of HepA vaccination among young children. Additional education of providers, particularly FMs, might be beneficial. Increasing HepA vaccination among children aged 12 to 23 months is an important strategy to limit transmission of HAV to adults and to increase the pool of adolescents and adults who are immune to HAV, because HAV infection is more severe in this age group.1,3,4
Physician barriers to strongly recommending and offering HepA vaccination to all 1- to 2-year-old patients should be identified and interventions implemented to address them. Considering decreasing anti-HAV seroprevalence in adults and poor 2-dose HepA vaccine coverage overall, it is important for physicians to follow the ACIP recommendations to vaccinate children aged 12 to 23 months and consider catch-up vaccination to improve population protection from HAV.
What’s New.
Gaps in hepatitis A virus infection and hepatitis A recommendations knowledge exist among family medicine physicians and pediatricians. Whereas 92% of pediatricians strongly recommend hepatitis A vaccine for children 1- to 2-years-old, only 59% of family medicine physicians recommend the vaccine to this age group.
Acknowledgments
We thank Lynn Olson, PhD, and Karen O’Connor from the Department of Research, AAP, Bellinda Schoof, MHA, at the AAFP, and the leaders of the AAP and AAFP for collaborating in the establishment of the sentinel networks in pediatrics and family medicine. We also thank all pediatricians and FMs in the networks for participating and responding to this survey.
Financial disclosure: This work was supported by a grant from the US Centers for Disease Control and Prevention SIP (5U48DP0011938) through the Rocky Mountain Prevention Research Center. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the US Centers for Disease Control and Prevention.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Centers for Disease Control and Prevention. Surveillance for Viral Hepatitis-United States. 2014 Available at: http://www.cdc.gov/hepatitis/Statistics/2014Surveillance/index.htm. Accessed May 24, 2016.
- 2.Murphy TV, Denniston MM, Hill HA, et al. Progress toward eliminating hepatitis A disease in the United States. MMWR Suppl. 2016;65:29–41. doi: 10.15585/mmwr.su6501a6. [DOI] [PubMed] [Google Scholar]
- 3.Collier MG, Tong X, Xu F. Hepatitis A hospitalizations in the United States, 2002–2011. Hepatology. 2014;61:481–485. doi: 10.1002/hep.27537. [DOI] [PubMed] [Google Scholar]
- 4.Ly KN, Klevens RM. Trends in disease and complications of hepatitis A virus infection in the United States, 1999–2011: a new concern for adults. J Infect Dis. 2015;212:176–182. doi: 10.1093/infdis/jiu834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) [erratum in: 1997;46:588] MMWR Recomm Rep. 1996;45:1–30. [PubMed] [Google Scholar]
- 6.Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 1999;48:1–37. [PubMed] [Google Scholar]
- 7.Advisory Committee on Immunization Practices (ACIP) Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55:1–23. [PubMed] [Google Scholar]
- 8.Hill HA, Elam-Evans LD, Yankey D, et al. Vaccination coverage among children aged 19–35 months - United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:1065–1071. doi: 10.15585/mmwr.mm6539a4. [DOI] [PubMed] [Google Scholar]
- 9.Department of Health and Human Services Centers for Disease Control and Prevention, Advisory Committee on Immunization Practices (ACIP) Summary report. 2014 Oct 29–30; Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/min-archive/min-2014-10.pdf. Accessed April 2, 2016.
- 10.Klevens RM, Denniston MM, Jiles-Chapman RB, et al. Decreasing immunity to hepatitis A virus infection among US adults: findings from the National Health and Nutrition Examination Survey (NHANES), 1999–2012. Vaccine. 2015;33:6192–6198. doi: 10.1016/j.vaccine.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Singleton RJ, Hess S, Bulkow LR, et al. Impact of a statewide childhood vaccine program in controlling hepatitis A virus infections in Alaska. Vaccine. 2010;28:6298–6304. doi: 10.1016/j.vaccine.2010.06.113. [DOI] [PubMed] [Google Scholar]
- 12.Williams WW, Lu PJ, O’Halloran A, et al. Surveillance of vaccination coverage among adult populations - United States, 2014. MMWR Surveill Summ. 2016;65:1–36. doi: 10.15585/mmwr.ss6501a1. [DOI] [PubMed] [Google Scholar]
- 13.Crane LA, Daley MF, Barrow J, et al. Sentinel physician networks as a technique for rapid immunization policy surveys. Eval Health Prof. 2008;31:43–64. doi: 10.1177/0163278707311872. [DOI] [PubMed] [Google Scholar]
- 14.McMahon SR, Iwamoto M, Massoudi MS, et al. Comparison of email, fax, and postal surveys of pediatricians. Pediatrics. 2003;111:e299–e303. doi: 10.1542/peds.111.4.e299. [DOI] [PubMed] [Google Scholar]
- 15.Immunization Action Coalition. Hepatitis A prevention mandates for daycare and K-12. 2016 Available at: http://www.immunize.org/laws/hepa.asp. Accessed March 20, 2016.
- 16.Dorell CG, Yankey D, Byrd KK, et al. Hepatitis A vaccination coverage among adolescents in the United States. Pediatrics. 2012;129:213–221. doi: 10.1542/peds.2011-2197. [DOI] [PubMed] [Google Scholar]
- 17.Santoli JM, Rodewald LE, Maes EF, et al. Vaccines for children program, United States, 1997. Pediatrics. 1999;104:e15. doi: 10.1542/peds.104.2.e15. [DOI] [PubMed] [Google Scholar]
- 18.Rosenblatt RA. A view from the periphery: health care in rural America. N Engl J Med. 2004;351:1049–1051. doi: 10.1056/NEJMp048073. [DOI] [PubMed] [Google Scholar]
- 19.Lopez AS, Kolasa MS, Seward JF. Status of school entry requirements for varicella vaccination and vaccination coverage 11 years after implementation of the varicella vaccination program. J Infect Dis. 2008;197(suppl 2):S76–S81. doi: 10.1086/522139. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Vaccination coverage among children enrolled in Head Start programs and licensed child care centers and entering school–United States and selected reporting areas, 1999–2000 school year. MMWR Morb Mortal Wkly Rep. 2001;50:847–855. [PubMed] [Google Scholar]
- 21.The Community Guide. Vaccination programs in schools and organized child care centers. Available at: http://www.thecommunityguide.org/vaccines/schools_childcare.html. Accessed April 2, 2016.
- 22.New York City Department of Health and Mental Hygiene. Zucker JR Communication. 2015 Available at: https://www1.nyc.gov/assets/doh/downloads/pdf/imm/hepa-vac.pdf. Accessed September 10, 2016.


