Abstract
Although currently still the gold standard treatment for post-prostatectomy urinary incontinence, the artificial urinary sphincter (AUS) (AMS800) is an invasive procedure with associated risks factors. In this paper, we aim to outline what the scientific literature and what we personally believe are the factors that are useful and/or necessary to mitigate these risks, including both patient factors and surgeon factors. We also review special populations, including transcorporal (TC) AUS approach, AUS with inflatable penile prosthesis, AUS after male urethral sling, AUS erosion management, and AUS after orthotopic urinary diversion.
Keywords: Radiation, male, urethroplasty, prosthesis, urinary incontinence
Patient selection and workup
Cystoscopy
Cystoscopy prior to artificial urinary sphincter (AUS) is recommended and is considered mandatory (1). Cystoscopy excludes bladder pathology, indicates functional bladder capacity, and rules out urethral or bladder neck stricture.
Bladder capacity
Patients with poor capacity or poorly compliant detrusor musculature do poorly after AUS placement, at best requiring frequent voiding and at worst risking upper tract deterioration. Capacity may be addressed via cystoscopy or formal urodynamics (UDS).
Does UDS help?
Although UDS is optional, the authors have found it extremely useful in a minority of cases. Patients with mild OAB may have exacerbation of symptoms due to incompetent outlets, and a number of patients with OAB or some degree of urge incontinence may be treated after AUS with oral medication, neuromodulation techniques, or intravesical botulinum toxin.
What about a history of bladder cancer or unstable bladder neck contracture?
Patients who will need consistent cystoscopy or possible bladder/bladder neck interventions may receive AUS. To the extent possible, the disease process should be treated and remain as stable as possible. When evaluating the size and surgical technique, future instrumentation should be anticipated. The smaller sizes of cuffs (3.5 and 4.0 cm) will not open enough to consistently safely allow passage of a 21 Fr sheath and so should be avoided, even if a transcorporal (TC) or more proximal periurethral cuff must be placed.
Radiation or other high-risk factors
Patient risk factors—specifically pelvic radiation, prior AUS erosion, history of urethral stent placement, neurogenic bladder dysfunction and previous urethroplasty—have been responsible for an increased risk of AUS complications, specifically infection and erosion (2). Some studies have also suggested that patients with additional medical comorbidities, including hypertension, coronary artery disease, and low testosterone are more likely to develop erosion of their AUS (3,4). Table 1 lists patient, surgeon, and device dependent risk factors for AUS failure.
Table 1. Risk factors for artificial urinary sphincter failure.
| Patient dependent |
| Age |
| Insufficient manual dexterity |
| Mental inaptitude and/or motivation |
| Higher ASA-classification |
| Perioperative anticoagulative therapy |
| Diabetes mellitus |
| Coronary artery disease, hypertension |
| Hypogonadal state |
| Neurogenic bladder dysfunction |
| Non-compliant or low capacity bladder |
| Recurrent urinary tract infections |
| Radiation therapy |
| Prior surgery for SUI |
| Additional procedure during SUI surgery |
| Prior urethral surgery (e.g., urethroplasty) |
| Urethral atrophy |
| Compromised/frail urethra |
| History of urethral stent (UroLume®) |
| Iatrogenic factors (improper urethral catheterization and endoscopic manipulation with an active AMS800®) |
| Lack of education of non-urological staff |
| Surgeon dependent |
| Inadequate patient selection |
| Deficient training and experience |
| Lack of meticulous and sterile surgical technique |
| Low-volume center surgeon |
| Deficient preoperative preparation |
| Long operative time |
| Device dependent |
| Non-mechanical |
| Urinary retention |
| Erosion |
| 3.5 cm cuff (in high risk settings) |
| Tandem cuff |
| Erroneous cuff sizing (too large, too small) |
| Inadvertent device deactivation |
| Mechanical |
| System fluid leak |
| Insufficient reservoir pressure |
| Pump malfunction |
ASA, American Society of Anesthesiologists; SUI, stress urinary incontinence.
A prior AUS revision, especially for erosion or infection of the cuff, predisposes to another similar event. The reason for this occurrence is still unclear but it is likely that erosion reduces urethral blood supply due to scar tissue it induces. Hypertension and generalized artery disease may also add to the vascular insufficiency leading to tissue breakdown and urethral cuff erosion. This is the leading cause of early AUS revision, with sub-cuff urethral atrophy being the usual cause of late (>12 months) revision. Table 2 lists early and late causes of revision surgery for AUS.
Table 2. Causes of revision surgery for artificial urinary sphincter.
| Early |
| Erroneous cuff sizing (leading to persistent incontinence or urinary retention) |
| Insufficient reservoir pressure (persistent urinary incontinence) |
| Discomfort at pump site |
| Unsatisfactory accessibility of scrotal pump |
| Erosion (often due to unrecognized urethral injury) |
| Late |
| Device mechanical malfunction (fluid leak, pump malfunction) |
| Sub-cuff urethral atrophy (leading to recurrent incontinence) |
| Infection |
| Erosion |
The role of prior radiation in subsequent cuff infection-erosion rate has been controversial in prior published literature. It is known that radiation causes long-term biological effects such as obliterative endarteritis of small vessels, which result in tissue ischemia, fibrosis, necrosis and abnormal tissue repair. These effects contribute to a higher likelihood of infection-erosion (2-9). The erosion rate is higher in patients receiving radiotherapy than in patients without a prior history of irradiation.
The management strategies for AUS revisions for infection-erosion should include placing the cuff at alternate cuff sites (more proximal or distal to the original one), TC approach (see below) and downsizing of cuff size. The use of the UroLume® stent (no longer available in the United States) across post-erosion stricture site has been recommended by some authors (10). However, other authors have reported dismal results (11).
Despite patient characteristics and risk factors, there are also surgeon-dependent factors. Not only patient selection is critical, but showering with a chlorhexidine soap starting several days prior to the procedure (12), a thorough scrub of the operating field, meticulous attention to sterile technique, scrupulous use of pre- and possibly peri-operative broad-spectrum antibiotics, avoidance of hematomas or excessive use of electrocautery, and copious irrigation of the surgical field are good surgical principles to which to adhere. Table 3 lists factors for minimizing risk of prosthetic infection. Delaying device activation for 4–6 weeks, and perhaps up to 8 weeks in patients with fragile urethras or following irradiation are generally followed practices. In high-risk patients, we may postpone reimplantation for up to 6 months to be sure that the urethra is completely healed. If a non-significant stricture develops, a direct vision internal urethrotomy may be attempted once; otherwise, the patient should undergo open urethroplasty. In our experience, one of the authors (FE Martins) always tries to perform synchronous urethral repair at the time of revisional surgery for cuff infection-erosion (see below). In most cases where the erosion is not circumferential, the urethra is not necrotic and the gap between both urethral ends is not too great, an anastomotic urethroplasty is feasible (data in preparation for publication). This approach, however, is controversial, and a multi-institutional retrospective study suggested that the type of repair at the time of erosion did not influence subsequent urethral stricture rates, although complete erosions were more likely to result in strictures compared to partial erosions (13). In our opinion, a urethral stent should be avoided at all costs, as we have never had any successful patient after such stent placed at the bulbar urethra or bladder neck followed by AUS implantation later (unpublished data).
Table 3. Factors for minimizing risk of prosthetic infection.
| Adequate patient selection |
| Sterile urine culture if possible |
| Adequate (preferably alcohol based) prep |
| Surgical shaving/clipping in operative room |
| Water-proof drapes and gowns |
| Double-gloving |
| Minimize OR traffic |
| Laminar flow equipped OR |
| Frequent antibiotic wound irrigation |
| Experienced implant surgeon/team |
| Meticulous surgical technique |
| Meticulous intraoperative hemostasis |
| Avoid long OR time |
| Peri-operative antibiotic prophylaxis |
OR, operation room.
Satisfaction with AUS is very high but reliant on patient expectations. An AUS would not be expected to restore the continence that the patient had prior to prostatectomy, nor will it relieve any underlying or de novo bladder dynamic changes, such as overactivity due to radiation cystitis. We counsel that patients typically achieve at least 80% improvement in their SUI symptoms and that each cuff or full revision generally leads to less improvement than the one preceding it.
Surgical steps
Positioning
The patient is placed in a high lithotomy position, positioned such that one leg may be lowered safely to allow for groin incision. Care is taken to make sure legs are well padded and that the knees are angled <90 degrees. Hair removal should be done on the operative table. Skin preparation should ideally incorporate alcohol and chlorhexidine.
Positioning and surgical approach
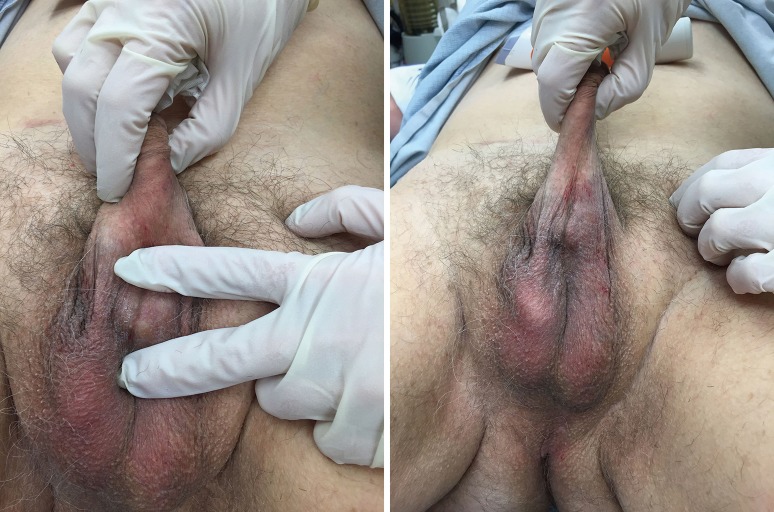
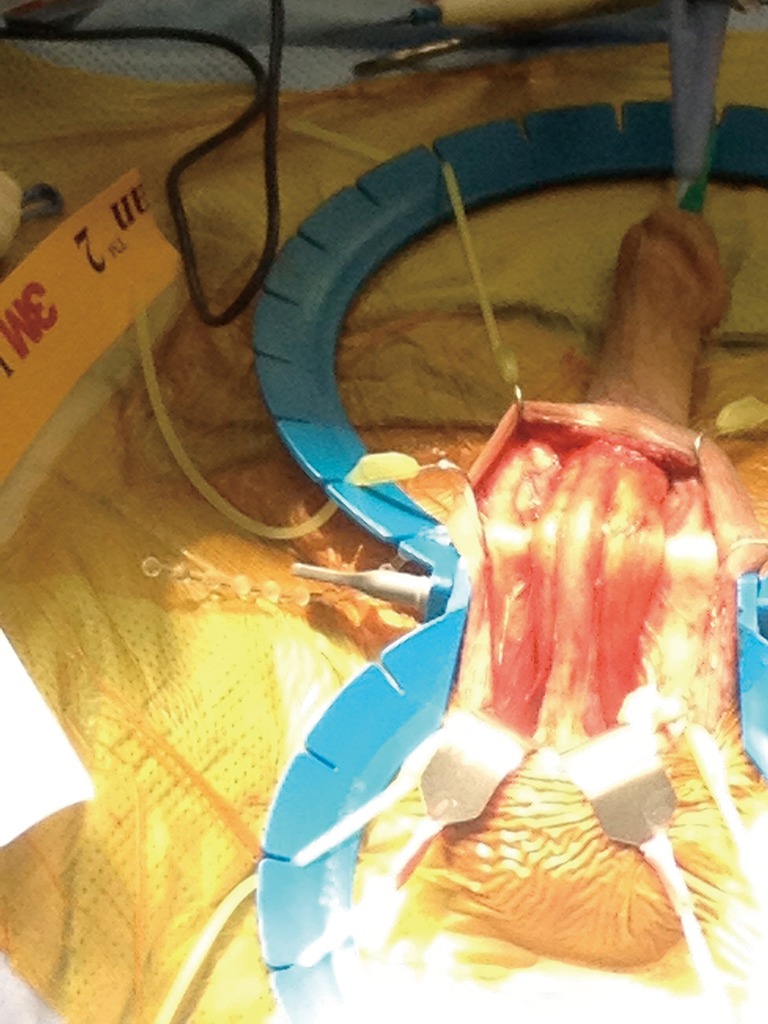
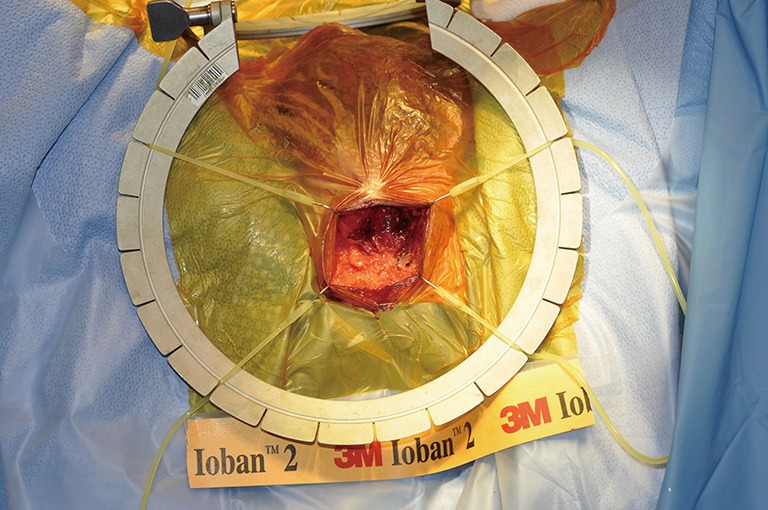
Although the penoscrotal has been touted as an easier approach, especially for those who do not perform AUS frequently, the sites chosen tend to be more distal on the urethra, of smaller size, and fail earlier (14) (Figures 1,2). A perineal approach will allow full access to the proximal, mid, and distal bulbar urethra, and is appropriate for single or double cuff placement, as well as periurethral and TC approaches (Figure 3-5).
Figure 1.
Exposure of the bulbar urethra via penoscrotal approach.
Figure 2.
Cuff placed too distally, at penoscrotal junction.
Figure 3.
Incision through perineal fat.
Figure 4.
Demonstration of the bulbar urethra.
Figure 5.
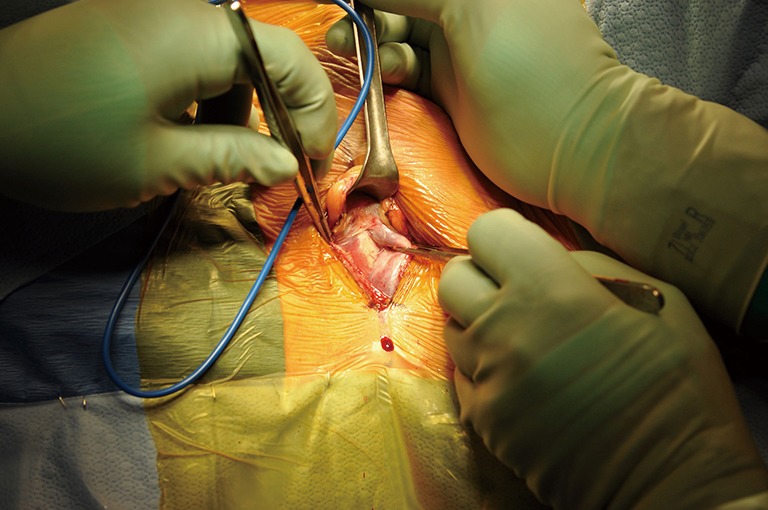
Initial dissection for a periurethral cuff placement.
A catheter is placed; typically, a 12 Fr silicone catheter is used if the surgeon is not planning to remove it during the case for sizing. The meatus and fossa are irrigated using an antibiotic solution.
If a no-touch approach is desired, occlusive sheets are placed over the skin after markings are made, including a midline perineal raphe mark and the chosen site for the pressure regulating balloon.
The raphe incision is made, and care is taken to stay in the midline. Once the bulbospongiosus muscle in encountered, typically at or just distal to the corporal crus, it is divided. The retractor of choice is placed, and the tissue adjacent to the urethra is divided sharply, with care to control any bridging vessels.
Cuff location(s) and considerations
Depending on age and comorbidities, it is likely that the AUS will require revision during the patient’s lifetime, often several times. For this reason, it is best to plan surgery with the option of later successful revisions. The major disadvantage of double cuff placement is that the wide area of urethra that is used will take away some future flexibility of placement (see below). In general, we recommend placing the AUS for the 1st time at the area of the crus, with subsequent cuffs placed in either proximally (at the area of the perineal body or more proximal) or distally (especially if a TC approach is used).
Dissection is carried around the urethra sharply, taking care to continue visualizing the dissection. It is better to err on the side of the corpora/septum rather than risk urethrotomy.
Sharp dissection vs. blunt
The urethra is eccentric within the bulb, with thin spongiosal tissue dorsally. The corpus spongiosum is densely adherent to the tunica albuginea (TA) in the area of the septum. If blunt dissection is performed, this may lead to urethral injury, and the dorsal urethra is the most common site of iatrogenic injury. If not recognized, this will lead to cuff erosion. For these reasons, we recommend sharp dissection, with care to visualize all aspects of the dissection until the urethra is completely encircled.
Once the urethra has been completely encircled, a vessel loop is placed and a window of 2 cm is created.
The urethral integrity is interrogated by introducing fluid alongside the catheter while the proximal urethra is compressed, to identify any leaks.
Urethral irrigation with proximal occlusion to demonstrate lack of urethral injury (Figure 6)
Figure 6.
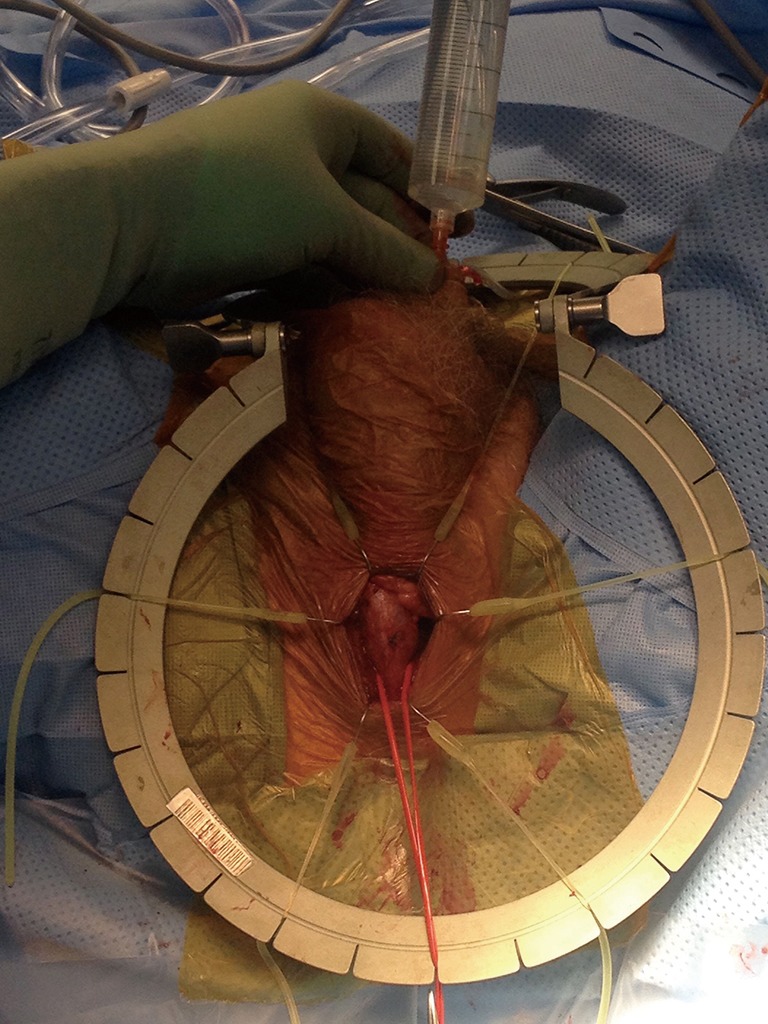
Urethral irrigation with proximal occlusion to demonstrate lack of urethral injury.
Despite meticulous dissection (never blunt!) of the dorsal attachment of the corpus spongiosum off of the TA, it is important to interrogate the urethra for subtle injuries. One author (WO Brant) has seen an iatrogenic injury that fistulized over several months when it was not recognized. The surgeon had irrigated the urethra but the hole caused by iatrogenic injury was so small that no fluid came out. However, the fistula manifested as a urinoma and was eventually diagnosed with occlusion of the urethra proximal to the cuff site. Intraoperatively, this can be accomplished by tensioning a vessel loop at the most proximal end of the dissection and putting fluid into the meatus, either with the catheter in or out. Alternatively, finger pressure can be used to occlude the proximal urethra. If an injury is noted, especially on the dorsal aspect of the urethra, the safest thing to do, especially for occasional implanters, is to leave a catheter in place for healing, stop the case, and do the surgery at a later date.
The measuring tape is used to decide on the size of the cuff. Although there is some controversy about what size to use, it is important that the urethra is measured accurately.
The cuff is placed, and positioned so that the patient will not be sitting on the tab of the cuff.
Single cuff vs. double vs. TC
Double cuff
Although placement of a double cuff (either at one surgery or added later as a staged procedure) is tempting due to initial improvements in continence, we have found that, ultimately, incontinence rates are equal and there is a higher rate of revision and erosion, particularly of the distal cuff (15,16). Additionally, the two cuffs remove some location flexibility for placement of future cuffs. For these reasons, we recommend single cuff placement if at all possible.
TC placement (Figures 7-15)
Figure 7.
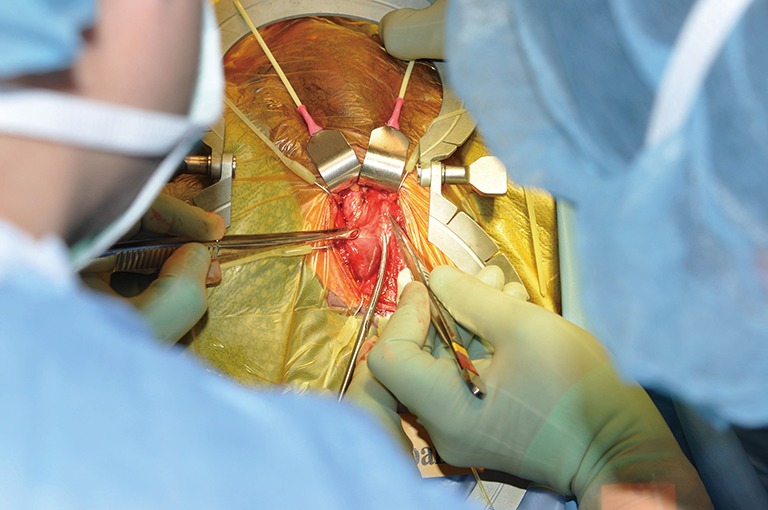
Clearing off the tunica albuginea (TA).
Figure 8.
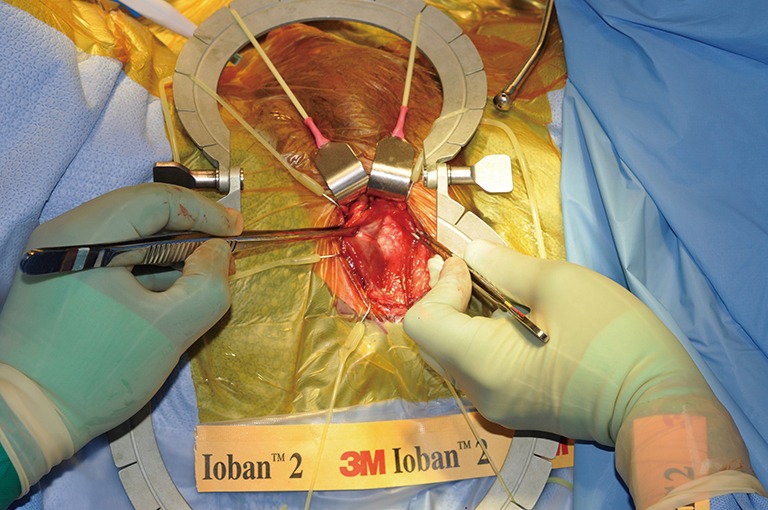
Exposure of the TA. TA, tunica albuginea.
Figure 9.
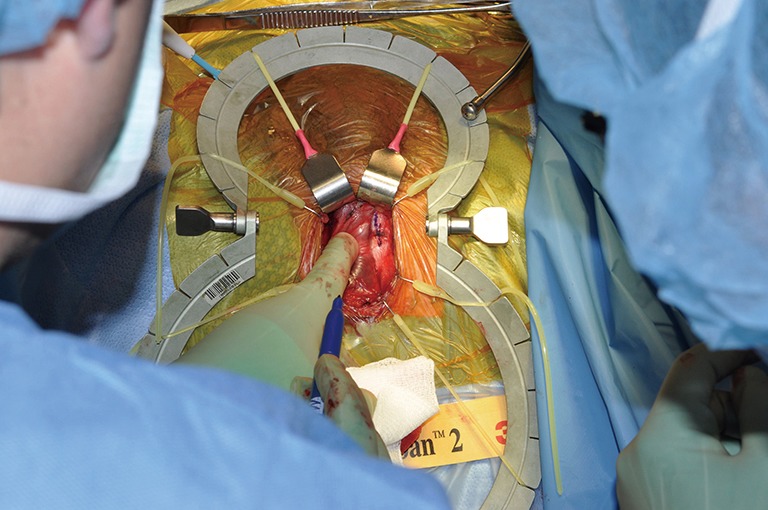
Marking the TA. TA, tunica albuginea.
Figure 10.
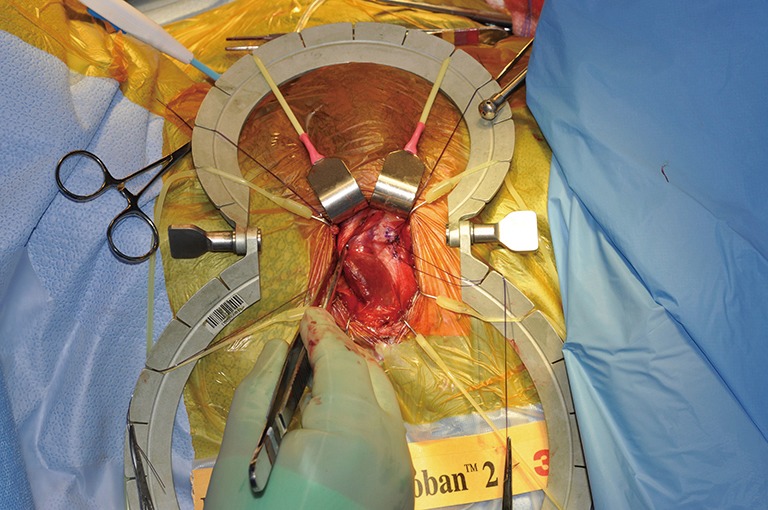
Placing sutures lateral to the proposed corporotomy.
Figure 11.
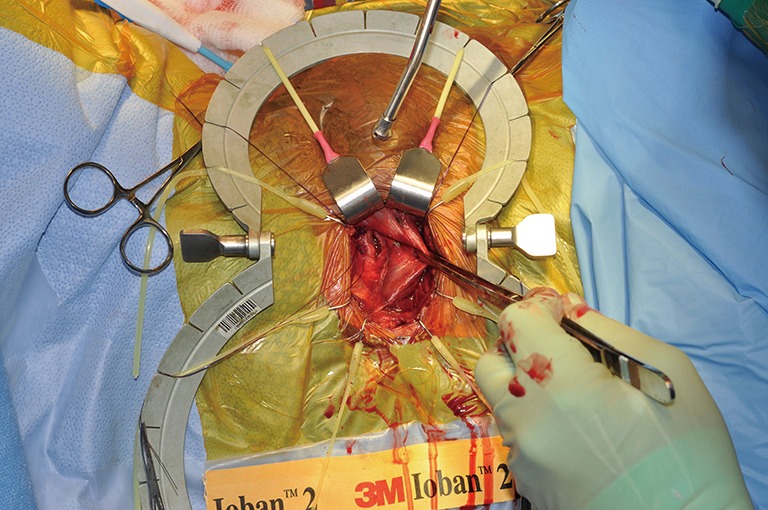
2 cm corporotomy.
Figure 12.
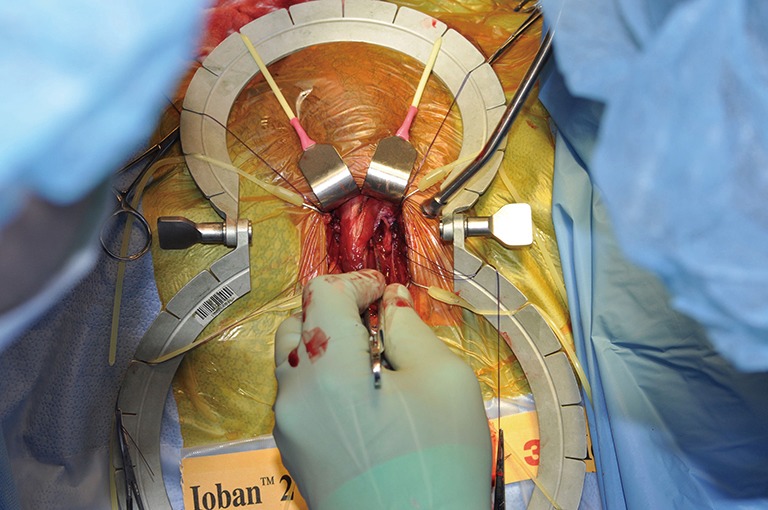
Contralateral corporotomy.
Figure 13.
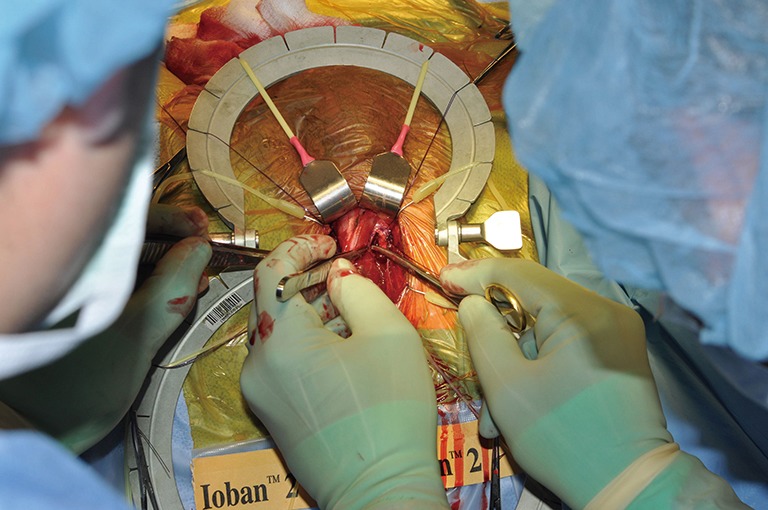
Starting the dissection under the TA. TA, tunica albuginea.
Figure 14.
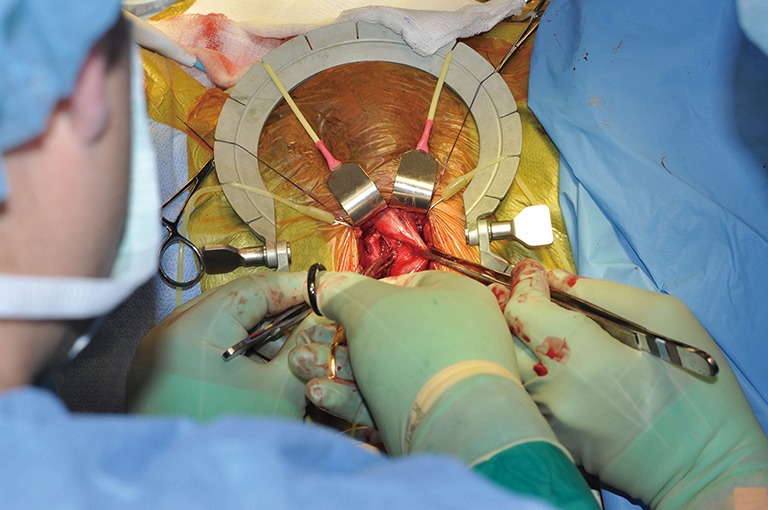
Continuing the dissection under the TA from the contralateral side. TA, tunica albuginea.
Figure 15.
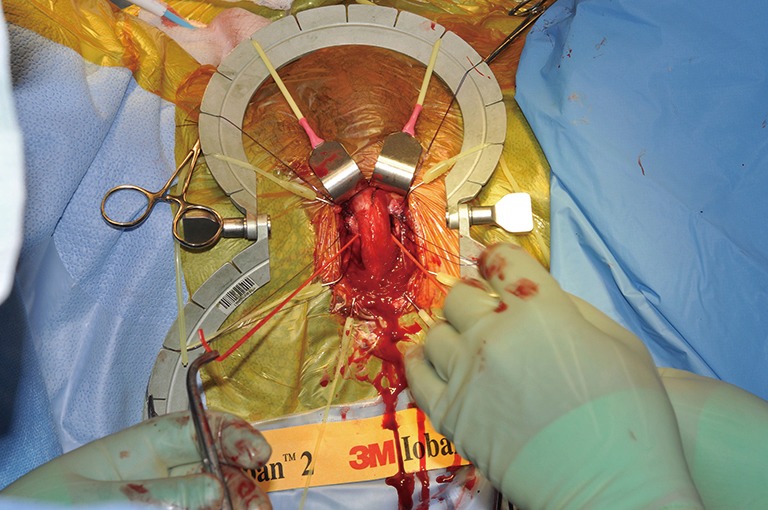
Both sides are now dissected and a vessel loop has been passed through.
The TC approach has several advantages and disadvantages, both practical and theoretical. Since a flap of TA is used to separate the dorsal urethra from the cuff, urethral injury is less likely. However, the relative lack of compressibility of the TA makes sizing of the cuff even more important than in the traditional periurethral approach and there is a higher incidence of urinary retention after the TC approach, especially in older patients (17). Theoretically, the TC approach may cause loss of erectile rigidity. This has been suggested via one patient (18) but, if the tunica is closed (as it should be to avoid postoperative hematoma), there may not be such an effect. Anecdotally, we have patients who initially expressed no interest in sexual activity but, once continent, became more interested. Intracavernosal therapy or penile prosthesis may be used successfully.
Attention is turned to the groin. An incision is made, typically in the right inguinal area along the skin lines, although it is often done in the midline. The incision is carried down until the transversalis fascia is encountered. It is either divided or pierced after the bladder is emptied. If the hiatus is big enough to not secure the PRB, sutures are preplaced for closure. The PRB is placed and inflated to 25 cc.
Pressure-regulating balloon (PRB) placement
Generally, the PRB has been placed in locations deep to the transversalis fascia. This may be done either via a midline or inguinal approach. Because of the small size and relative compressibility of the PRB compared to an inflatable penile prosthesis reservoir, if an ectopic (i.e., superficial to the transversalis fascia) location is chosen, the neck of the PRB must be secured to prevent herniation.
A spot is chosen in the dependent scrotum, typically on the patient’s dominant side. The scrotum is inverted into the groin incision, the dartos layer is dissected off, and the pump is placed in its site and guided back into place. A Babcock clamp is placed gently around the tubing to prevent pump migration during tubing connections.
Pump placement
The pump will need to be accessible and easy to manipulate several times per day and as such it is important to place it in a superficial location. Generally, this would be on the patient’s dominant hand side (although this is not critical) and in a low position in the scrotum in a subdartos pouch. We have found that, with postoperative swelling or hematoma, the pump can migrate cephalad and make manipulation more difficult. Therefore, we recommend that the patient manipulate the device by pulling gently on it during the postoperative period to maintain its location.
Attention is returned to the perineum. The tubing passer is attached to the tubing and, optionally, tunneled through the bulbospongiosus muscle prior to carefully passing the tubing to the groin.
Connections are carefully made, with care to not introduce any blood or debris into the tubing.
The pump is cycled several times and then deactivated, with care to leave enough fluid in the pump to make activation easy and painless.
The perineum is closed in 4 layers (bulbospongiosus muscle, fat, Colles fascia, skin), while the groin is closed in 3–4 layers.
Dressing are applied. The groin incision is closed in several layers. Typically, no drain is used other than the small catheter that is left overnight.
Postoperative care
Leave the system deactivated for 6 weeks
Although patients may desire early activation, it is important to leave the cuff in a deactivated position for at least 6 weeks postoperatively. Exceptions to this are only: (I) if the PRB itself is changed without any other procedures; and (II) if the cuff is downsized and the same pseudocapsule space is used.
A urethral catheter should be in overnight only or for as little time as possible.
After several days, an indwelling catheter will be colonized and has a high chance of leading to a urinary tract infection. As such, catheters should be used for as little time as possible. These catheters should be small enough to not cause pressure between the urethra and the deactivated cuff and thus should never be larger than 14 Fr in size. Retention may occur, especially if the TC approach is used in older men (see above).
Combined versus staged IPP and AUS implantation
Although most urologists favor separate implantation due to inherent risks, simultaneous dual implantation of an AUS and an inflatable penile prosthesis for urinary incontinence and erectile dysfunction through a single (transverse scrotal) or 2 incisions is safe, efficient and cost-effective. Moreover, when indicated or necessary, dual implantation may prove practical and rational. AUS and IPP synchronous placement by one or two incisions is feasible and safe and as effective as the 2-stage procedure, with better acceptance by patients. However, because of increased operative time, the infection rate is expected to be slightly increased, thus preventing widespread acceptance of the simultaneous implantation procedure. The literature on this topic is scant (19-21). More studies are needed in order to fully recommend this surgical strategy to both urologists and their patients under a single anesthetic event. The authors have performed dual implantation with good outcomes and, therefore, recommend this strategy in selected cases, such as patients unwilling to undergo 2 separate anesthetic events or those who live far away and, therefore, would like to avoid travels as long as the patients fully understand the potential of increased risk of infection. If a staged approach is chosen, it is typical to place the AUS first, to achieve continence and make the possibility of sexual interest and success more realistic, prior to IPP placement.
What about IPP if TC approach is used (Figure 16)?
Figure 16.
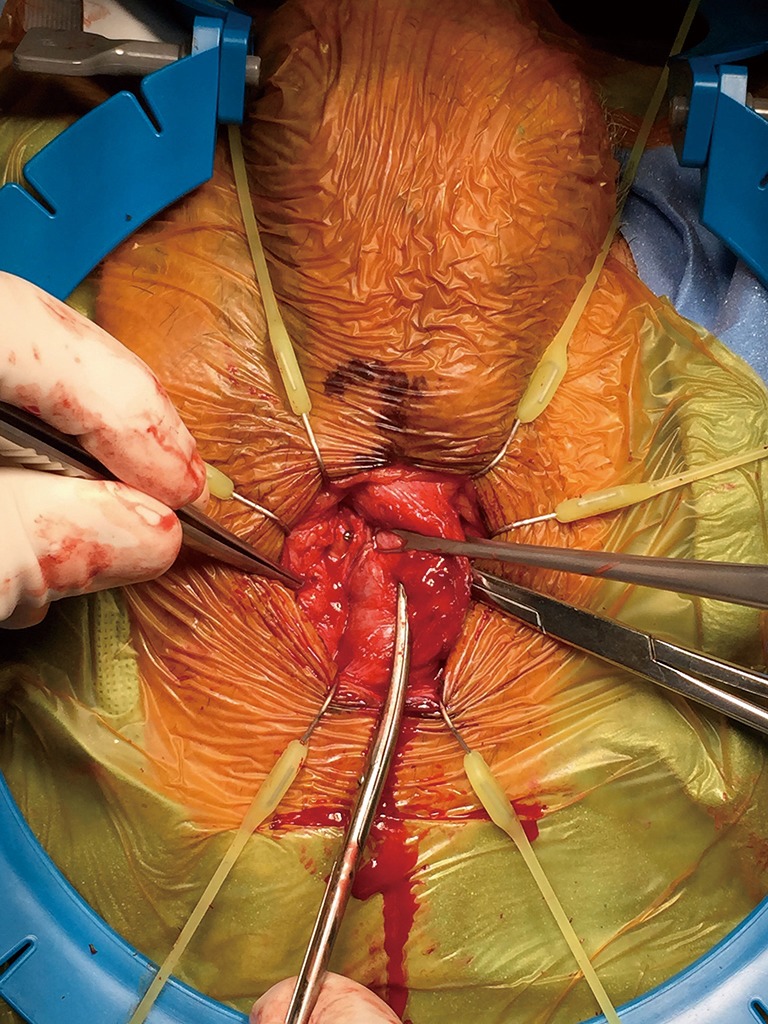
Transcorporal dissection with an IPP in situ. The pseudocapsule of the left IPP cylinder is visualized.
TC placement of the AUS cuff can restore urinary continence, but concern exists about the safety of placement of an inflatable penile prosthesis later. The one and single study we could find in the literature was presented at the AUA Annual Meeting in 2008 where the authors reported successful outcomes in 3 patients who underwent this surgical reconstruction (22). However, we would definitely not recommend this reconstruction for the occasional implanter or otherwise small volume centers. It is important to try to avoid going through the pseudocapsule of the IPP (see photo) and thus prevent the rubbing of silicone on silicone. If necessary, one of the authors (WO Brant) has placed an interposition xenograft between the IPP and the AUS cuff (Figure 17).
Figure 17.
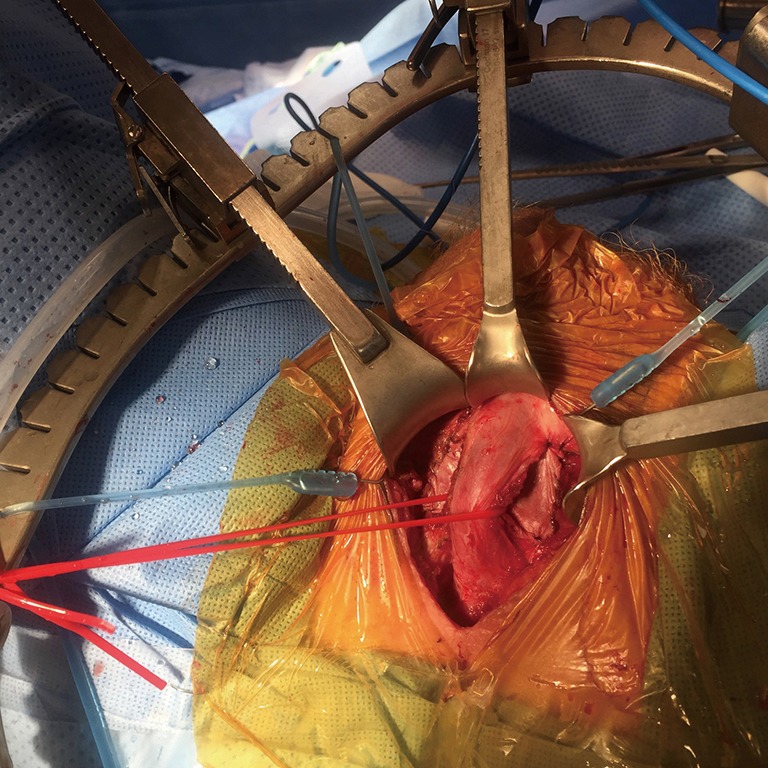
A xenograft has been placed as a barrier in a case where the preexisting IPP pseudocapsule was violated in a transcorporal approach.
AUS after male urethral sling
Although the AMS 800 remains the gold standard treatment option for male non-neurogenic urinary incontinence, urethral slings have also been recommended in the last 2 decades. Since then multiple sling varieties have appeared on the market. Despite success in appropriately selected candidates, up to 20% of males treated with a male urethral sling for stress urinary incontinence may experience a decline in their degree of improvement over time (11,23). In these patients with persistent or recurrent urinary incontinence, implantation of an AUS is desirable in most cases. If a patient elects an AUS to treat his incontinence after a failed sling, the prospects for a successful outcome are high. However, there is little information available to counsel this set of patients on this treatment option (24-26). In a retrospective analysis comparing outcomes after AUS vs. repeat sling after prior failed male urethral sling, the authors identified a 7-fold difference in the rate of persistent incontinence in favor of primary AUS placement (27). In conclusion, primary AUS placement remains a viable and safe therapeutic option for men with persistent or recurrent stress urinary incontinence after a previously failed male sling and, therefore, it should be recommended in this situation.
Issues regarding synchronous urethral reconstruction and revision for AUS cuff infection-erosion (Figures 18,19)
Figure 18.
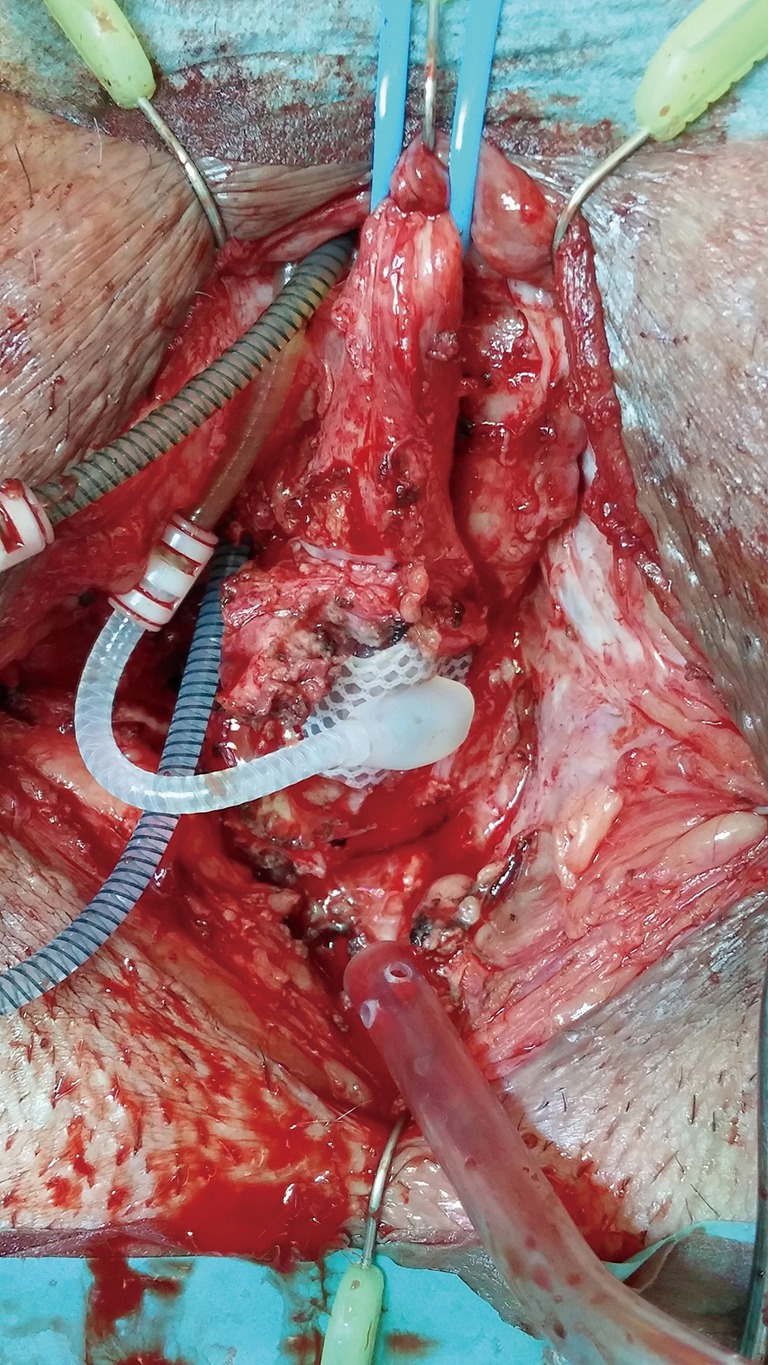
Urethral erosion within the cuff pseudocapsule.
Figure 19.
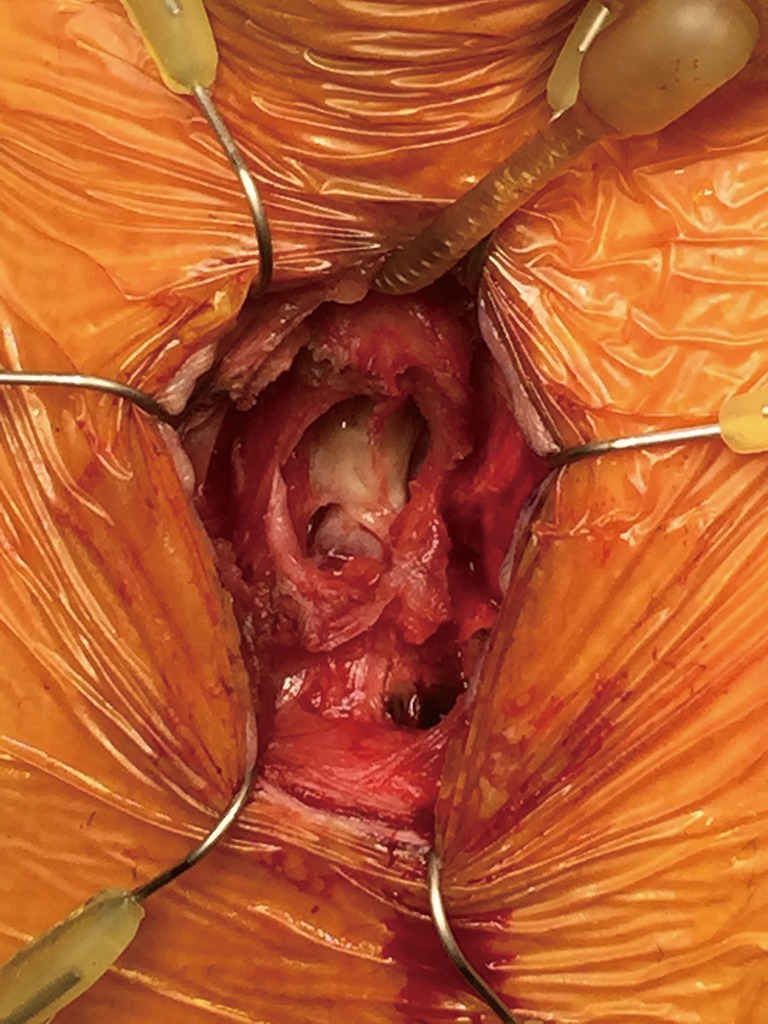
Cuff erosion into the urethra.
Traditionally, AUS cuff infection-erosion has been managed by urinary diversion with indwelling urethral catheterization or suprapubic cystostomy for several weeks followed by AUS reimplantation after 4 to 6 months. Recently, it has been reported that simultaneous urethral reconstruction and AUS removal for cuff infection-erosion may prevent bulbar urethral stricture development, thus facilitating AUS replacement later (28). This concept is attractive since it theoretically may reduce the risk of a urethral stricture, which in turn will imply a 3-stage procedure: removal of the eroded cuff, urethroplasty and, finally, placement of a new AUS device with its attendant delay for return to normality. However, other data (13) suggests that the type of repair (simple catheter drainage, urethrorrhaphy, urethroplasty, etc.) at the time of erosion did not influence subsequent urethral stricture rates, although complete erosions were more likely to result in strictures compared to partial erosions. Several caveats need to be discussed. This patient population is quite heterogenous, making patient and criteria selection for this strategy somewhat difficult and biased. The local tissue conditions encountered at the time of cuff removal vary from mild inflammatory reaction associated with partial erosion to a much more severe circumferential erosion and more densely fibrotic tissue, to even a catastrophic devastation of the urethra with necrosis, purulence, and significant loss of the bulbar urethra and local surrounding perineal tissues. Lastly, because patient randomization is difficult, the surgical technique adopted for the management of these patients is also variable, ranging from simple surgical debridement and urethrorrhaphy to formal anastomotic repair or eventually temporary perineal urethrostomy. Nevertheless, we also have found in our series that in cases where the urethral segment affected has not suffered severe damage and thus is deemed amenable to immediate repair, we have followed the same strategy of synchronous reconstruction (29). Nonetheless, it is important to bear in mind that these urethras are at higher risk for complications such as stricture formation and atrophy due to the inherent compromise of the blood supply (Figures 20,21).
Figure 20.
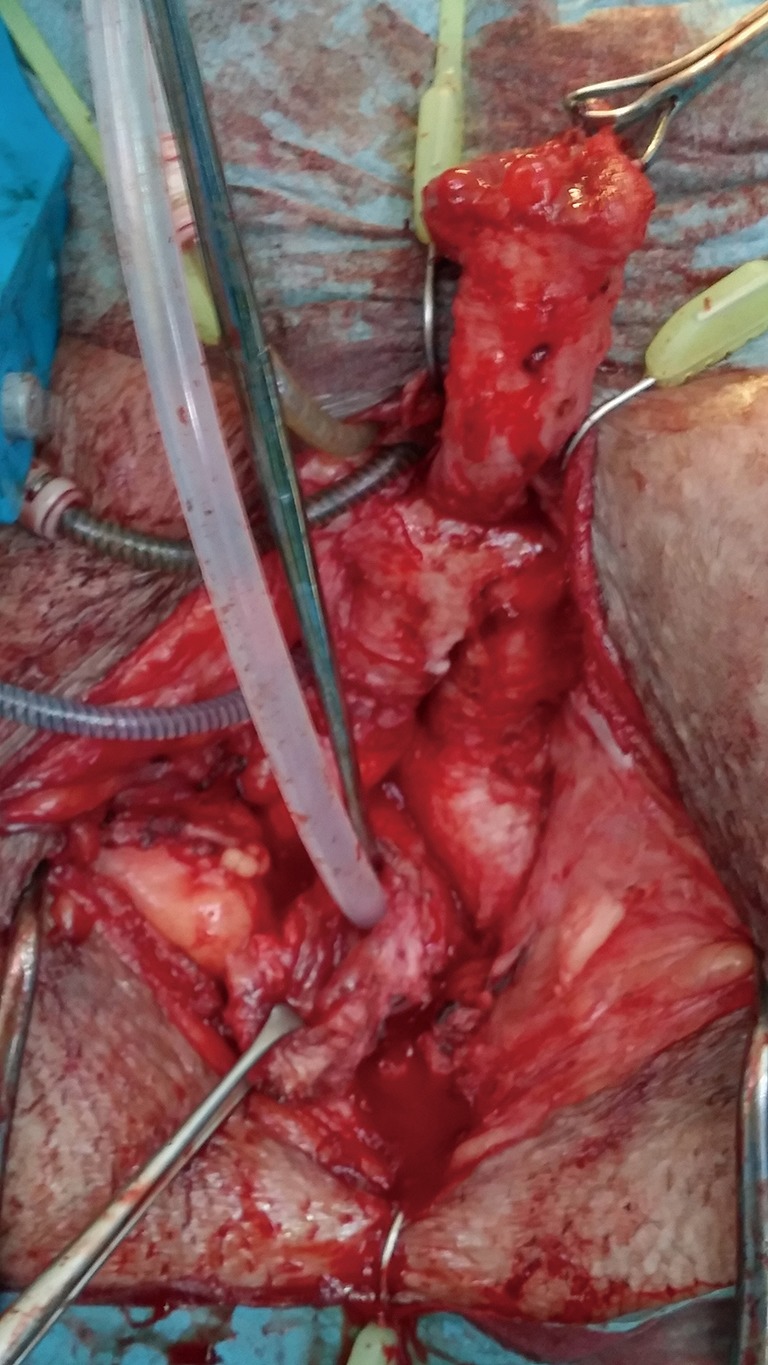
Cuff removed and erosion site excised.
Figure 21.
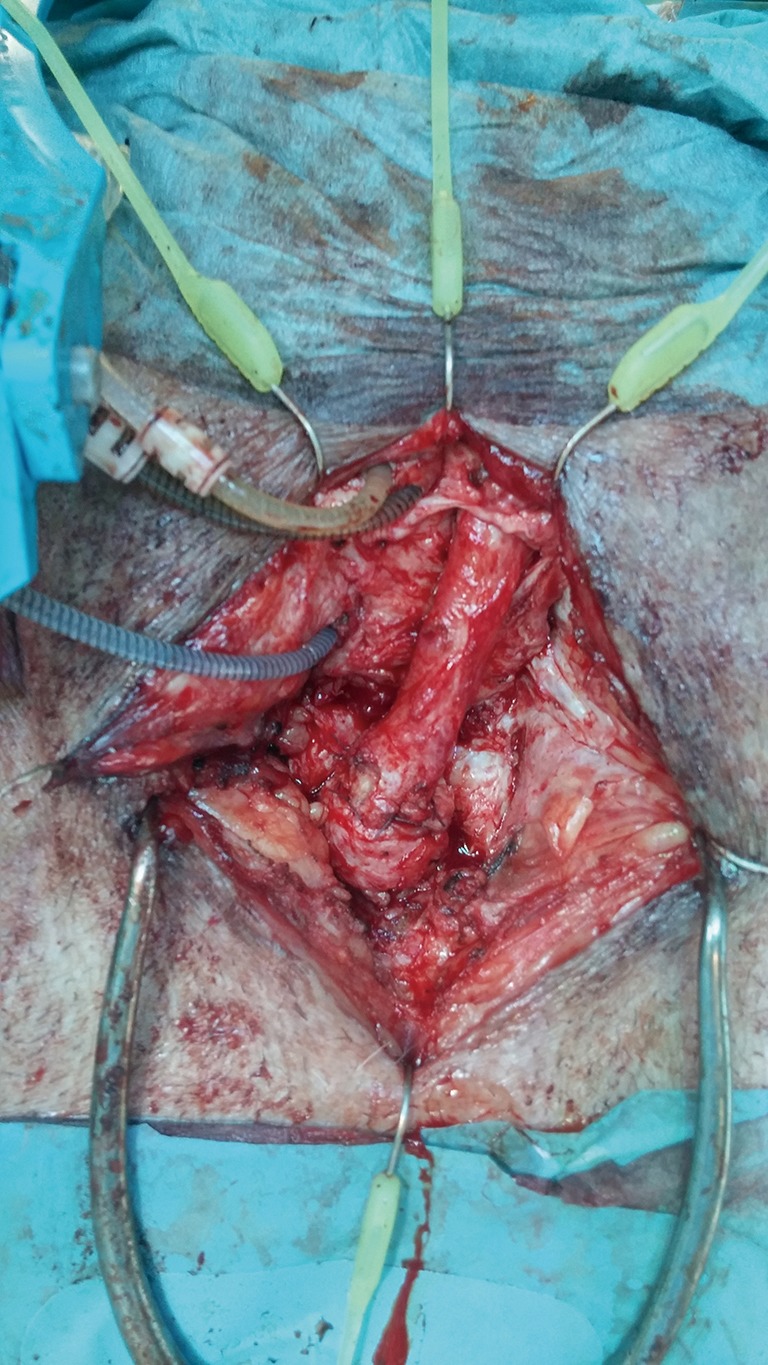
Completion of anastomotic urethroplasty after erosion.
AUS placement after radical cystectomy and orthotopic neobladder urinary diversion
Stress urinary incontinence is a known iatrogenic possibility after radical cystectomy and orthotopic urinary reconstruction. Although orthotopic neobladders have become the standard form of urinary diversion after radical cystectomy at many institutions over the last 25 years, few studies have focused on the use of AUS for the treatment of urinary incontinence after radical cystectomy and orthotopic reconstruction. To our knowledge, O’Connor et al were the first to report on the use of the AUS in patients after radical cystectomy and orthotopic neobladder (30). In a USC retrospective study published in 2013, of a total 64 patients that underwent AUS placement after radical cystectomy and orthotopic neobladder from 1994 to 2009, only 36 were evaluated in this study (31). The implantation procedure was standard, the reservoir being placed extraperitoneally through a separate incision approximately two finger breadths medial to the anterior superior ischial spine. Device deactivation was kept for 4–6 weeks postoperatively and patients with elevated postvoid residual volumes were taught how to perform clean intermittent catheterization after AUS deactivation. Potential risk factors for adverse outcomes regarding AUS placement and durability were evaluated: BMI, diabetes mellitus, chemotherapy and time interval between chemotherapy and AUS placement, pelvic irradiation, patient’s fitness/performance status and operative time. Urinary incontinence improved in 76% of patients. Overall revision rate of 60% was significantly high, which should not be surprising due to the high-risk factors inherent to this specific population of the study with an intestinal neobladder and eventually infected or persistently colonized urine, even further aggravated by the need of intermittent “clean” self-catheterization in some of them. A similar high complication rate is also known to occur in neurogenic bladders due to a high incidence of urine bacterial colonization, urinary tract infection, further worsened if clean intermittent catheterization is needed in these dysfunctional bladders.
Special consideration should be taken regarding reservoir placement in patients with an orthotopic neobladder. It is critical that the implanting surgeon should keep away from the midline suprapubic area to avoid inadvertent damage to the neobladder and ileal loops. While this space can be used for blind placement of the reservoir through a single transscrotal incision in post-radical prostatectomy patients, it should be avoided always in post-radical cystectomy and orthotopic neobladder patients. One of the authors (FE Martins) had this complication once (blind neobladder and ileal perforation leading to peritonitis and conversion to ileal conduit) which happened approximately 22 years ago, and it is so indelibly etched in his memory! Therefore, a separate incision as proposed by the authors of the USC study should always be recommended in this subset of patients.
All in all, the placement of an AUS for treatment of SUI after orthotopic neobladder is apparently reasonably safe, efficacious, well tolerated and reliable and has a favorable impact on patients’ quality of life. However, the revision rate for non-mechanical complications should be improved.
Acknowledgements
None.
Footnotes
Conflicts of Interest: Dr. Brant is a consultant, investigator, and grant recipient for Boston Scientific. FE Martins has no conflicts of interest to declare.
References
- 1.Biardeau X, Aharony S, AUS Consensus Group , Artificial Urinary Sphincter: Report of the 2015 Consensus Conference. Neurourol Urodyn 2016;35 Suppl 2:S8-24. 10.1002/nau.22989 [DOI] [PubMed] [Google Scholar]
- 2.Brant WO, Erickson BA, Elliott SP, et al. Risk factors for erosion of the artificial urinary sphincters: a multicenter prospective study. Urology 2014;84:934-8. 10.1016/j.urology.2014.05.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raj GV, Peterson AC, Webster GD. Outcomes following erosions of the artificial urinary sphincter. J Urol 2006;175:2186-90. 10.1016/S0022-5347(06)00307-7 [DOI] [PubMed] [Google Scholar]
- 4.Hofer MD, Morey AF, Sheth K, et al. Low Serum Testosterone Level Predisposes to Artificial Urinary Sphincter Cuff Erosion. Urology 2016;97:245-9. 10.1016/j.urology.2016.04.065 [DOI] [PubMed] [Google Scholar]
- 5.Kretschmer A, Buchner A, Grabbert M, et al. Risk factors for artificial urinary sphincter failure: World J Urol 2016;34:595-602. 10.1007/s00345-015-1662-9 [DOI] [PubMed] [Google Scholar]
- 6.Lai HH, Boone TB. Complex artificial urinary sphincter revision and reimplantation cases -- how do they fare compared to virgin cases? J Urol 2012;187:951-5. 10.1016/j.juro.2011.10.153 [DOI] [PubMed] [Google Scholar]
- 7.Martins FE, Boyd SD. Artificial urinary sphincter in patients following major pelvic surgery and/or radiotherapy: are they less favorable candidates? J Urol 1995;153:1188-93. 10.1016/S0022-5347(01)67547-5 [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Hadley H. Experiences with the artificial urinary sphincter in the irradiated patient. J Urol 1992;147:612-3. 10.1016/S0022-5347(17)37320-2 [DOI] [PubMed] [Google Scholar]
- 9.Walsh IK, Williams S, Mahendra V, et al. Artificial urinary sphincter implantation in the irradiated patient: safety, efficacy and satisfaction. BJU Int 2002;89:364-8. 10.1046/j.1464-4096.2001.01759.x [DOI] [PubMed] [Google Scholar]
- 10.Anger JT, Raj GV, Delvecchio FC, et al. Anastomotic contracture and incontinence after radical prostatectomy: a graded approach to management. J Urol 2005;173:1143-6. 10.1097/01.ju.0000155624.48337.a5 [DOI] [PubMed] [Google Scholar]
- 11.Li H, Gill BC, Nowacki AS, et al. Therapeutic durability of the male transobturator sling: mid-term patient-reported outcomes. J Urol 2012;187:1331-5. 10.1016/j.juro.2011.11.091 [DOI] [PubMed] [Google Scholar]
- 12.Magera JS, Jr, Inman BA, Elliott DS. Does preoperative topical antimicrobial scrub reduce positive surgical site culture rates in men undergoing artificial urinary sphincter placement? J Urol 2007;178:1328-32; discussion 1332. 10.1016/j.juro.2007.05.146 [DOI] [PubMed] [Google Scholar]
- 13.Gross MS, Broghammer JA, Kaufman MR, et al. Urethral Stricture Outcomes After Artificial Urinary Sphincter Cuff Erosion: Results From a Multicenter Retrospective Analysis. Urology 2017;104:198-203. 10.1016/j.urology.2017.01.020 [DOI] [PubMed] [Google Scholar]
- 14.Henry GD, Graham SM, Cornell RJ, et al. A multicenter study on the perineal versus penoscrotal approach for implantation of an artificial urinary sphincter: cuff size and control of male stress urinary incontinence. J Urol 2009;182:2404-9. 10.1016/j.juro.2009.07.068 [DOI] [PubMed] [Google Scholar]
- 15.O'Connor RC, Gerber GS, Avila D, et al. Comparison of outcomes after single or double-cuff artificial urinary sphincter insertion. Urology 2003;62:723-6. 10.1016/S0090-4295(03)00572-7 [DOI] [PubMed] [Google Scholar]
- 16.O'Connor RC, Lyon MB, Guralnick ML, et al. Long-term follow-up of single versus double cuff artificial urinary sphincter insertion for the treatment of severe postprostatectomy stress urinary incontinence. Urology 2008;71:90-3. 10.1016/j.urology.2007.08.017 [DOI] [PubMed] [Google Scholar]
- 17.Guralnick ML, Miller E, Toh KL, et al. Transcorporal artificial urinary sphincter cuff placement in cases requiring revision for erosion and urethral atrophy. J Urol 2002;167:2075-8; discussion 2079. 10.1016/S0022-5347(05)65088-4 [DOI] [PubMed] [Google Scholar]
- 18.Smith PJ, Hudak SJ, Scott JF, et al. Transcorporal artificial urinary sphincter cuff placement is associated with a higher risk of postoperative urinary retention. Can J Urol 2013;20:6773-7. [PubMed] [Google Scholar]
- 19.Kendirci M, Gupta S, Shaw K, et al. Synchronous prosthetic implantation through a transcrotal approach: an outcome analysis. J Urol 2006;175:2218-22. 10.1016/S0022-5347(06)00345-4 [DOI] [PubMed] [Google Scholar]
- 20.Linares E, Martinez-Salamanca JI, Portillo LD, et al. Simultaneous double implant: artificial urinary sphincter and penile prosthesis using scrotal unique incision. J Urol 2013;189:e373-4. 10.1016/j.juro.2013.02.481 [DOI] [Google Scholar]
- 21.Rolle L, Ceruti C, Sedigh O, et al. Surgical implantation of artificial urinary device and penile prosthesis through transcrotal incision for postprostatectomy urinary incontinence and erectile dysfunction: synchronous or delayed procedure? Urology 2012;80:1046-50. 10.1016/j.urology.2012.08.003 [DOI] [PubMed] [Google Scholar]
- 22.Donatucci CF, Buschemeyer WC, Webster GD. Penile prosthesis placement in men after transcorporal artificial urinary sphincter implantation. J Urol 2008;179:403. 10.1016/S0022-5347(08)61178-718076950 [DOI] [Google Scholar]
- 23.Zuckerman JM, Edwards B, Henderson K, et al. Extended outcomes in the treatment of male stress urinary incontinence with a transobturator sling. Urology 2014;83:939-45. 10.1016/j.urology.2013.10.065 [DOI] [PubMed] [Google Scholar]
- 24.Lentz AC, Peterson AC, Webster GD. Outcomes following artificial sphincter implantation after prior unsuccessful male sling. J Urol 2012;187:2149-53. 10.1016/j.juro.2012.01.119 [DOI] [PubMed] [Google Scholar]
- 25.Fisher MB, Aggarwal N, Vuruskan H, et al. Efficacy of artificial urinary sphincter implantation after failed bone-anchored male sling for post-prostatectomy incontinence. Urology 2007;70:942-4. 10.1016/j.urology.2007.07.022 [DOI] [PubMed] [Google Scholar]
- 26.Ziegelmann MJ, Linder BJ, Rivera ME, et al. The impact of prior urethral sling on artificial urinary sphincter outcomes. Can Urol Assoc J 2016;10:405-9. 10.5489/cuaj.3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ajay D, Zhang H, Gupta S, et al. The artificial urinary sphincter is superior to a secondary transobturator male sling in cases of a primary sling failure. J Urol 2014;191:734-8. [DOI] [PubMed] [Google Scholar]
- 28.Rozanski AT, Tausch TJ, Ramirez D, et al. Immediate urethral repair during explantation prevents stricture formation after artificial urinary sphincter cuff erosion. J Urol 2014;192:442-6. 10.1016/j.juro.2014.02.007 [DOI] [PubMed] [Google Scholar]
- 29.Martins FE, Marcelino JP, Sandul A, et al. Urethral reconstruction following artificial urinary sphincter cuff infection-erosion. Eur Urol Suppl 2016;15:e1156 10.1016/S1569-9056(16)61157-0 [DOI] [Google Scholar]
- 30.O’Connor RC, Kuznetsov DD, Patel RV, et al. Artificial urinary sphincter placement in men after cystectomy with orthotopic ileal neobladder: continence, complications, and quality of life. Urology 2002;59:542-5. 10.1016/S0090-4295(01)01655-7 [DOI] [PubMed] [Google Scholar]
- 31.Vainrib M, Simma-Chiang V, Boyd SD, et al. Potential risk factors and outcomes of artificial urinary sphincter placement after radical cystectomy and orthotopic neobladder urinary diversion. Neurourol Urodyn 2013;32:1010-3. 10.1002/nau.22345 [DOI] [PubMed] [Google Scholar]