Abstract
The enzymes activity, hydroxymethylfurfural (HMF) and amino acids in honeys are relatively low. However, they play very significant role for honey quality. In this study, enzymes, amino acids and HMF contents of Ethiopian monofloral honeys were investigated. Diastase, invertase and HMF were analyzed based on the Harmonized International Honey Commission method and amino acids using amino acids analyzer (HPLC). Diastase activity ranged from 3.91 ± 0.730 (Schefflera abyssinica) to 13.6 ± 2.30 [Becium grandiflorum (L: Lalibella)]; invertase 36.5 ± 1.93 (Leucas abyssinica) to 4.85 ± 2.36 (Schefflera abyssinica); and HMF 0 ± 0 (Hypoestes and Leucas abyssinica) to 3.37 ± 1.73 (Croton macrostachyus). Significant variations were observed among Schefflera abyssinica honeys in diastase content, despite being from the same botanical origin. Significant variations were also observed among Becium grandiflorum honeys in invertase and diastase contents. Bees’ geographical race and location affected enzymes activities. Lower level of enzymes could be an intrinsic characteristic of Ethiopian honey. Thus, enzymes activity alone cannot be a worthwhile indicator of quality for Ethiopian honey; besides diastase and invertase activity, the quality control of Ethiopian honeys should be supported by HMF parameters.
Keywords: Amino acid, Enzyme, Honey quality, Hydroxymethylfurfural, Monofloral, Ethiopia
Introduction
Honey is essentially a concentrated aqueous solution of different carbohydrates (70–80%). It also contains a very complex mixture of enzymes, amino acids, polyphenols, carotenoid-like substances, flavonoids and pollen grains (Ferreira et al. 2009; Lazarević et al. 2012). These constituents added by the bees and are derived from the plants (Ohashi et al. 1999; Jasicka-Misiak et al. 2012).
Enzymes, HMF and amino acids are relatively low in honey. Nevertheless, they play very important role for honey quality control. According to Codex Alimentarius and European Union, the HMF, diastase and proline content of a good quality and acceptable honey is <40 mg kg−1, ≥8 Schade scale and 180 mg kg−1 honey, respectively (Bogdanov 2009). Honey enzyme is affected by storage, and sensitive to temperature increase. Thus, enzyme content can be used as an indicator of storage time/freshness of honey. The activity of enzyme in honey also depends on age of the bees, stage of the colony, nectar flow, environmental conditions and the beekeeping practices (Karabournioti and Zervalaki 2001).
Amino acids in honey attributed mainly from pollen of the foraging plant and secretion of honeybees (Paramás et al. 2006). A study on forty-eight Spanish honey samples using HPLC indicated that proline was dominant in Eucalyptus and Orange blossom, and phenylalanine in Lavender and Thyme honeys (Hermosín et al. 2003).
HMF is a heterocyclic aldehydes, formed as a result of Maillard and caramelization reaction, most commonly derived from hexose degradation (Fallico et al. 2004). At room temperature, the action of normal honey acidity on reducing sugars can possibly produce HMF. On the other hand, the quantity of HMF increases upon heat treatment and storage. HMF has a toxic effect and also induces reactive oxygen species (De Smet et al. 2015). In vivo and in vitro study of Capuano and Fogliano (2011), and Arribas-Lorenzo and Morales (2010) reported that sulfotransferases metabolize HMF to its mutagenic derivative, sulfomethylfurfural, by sulfonation. Cytotoxicity of HMF to humans occurred by reduced granulocyte metabolism (Nässberger 1990). HMF is the most consistent marker of honey freshness or quality deterioration that is used by national, regional and international standards as quality control parameters (Gürkan and Altunay 2015).
International Honey Commission (IHC) set legislation to control the quality of honey. The most commonly used enzymes in quality control standards are diastase and invertase. These standards indicated that some monofloral honeys naturally have a lower diastase value due to their botanical origin and are accepted in international market (Bogdanov et al. 1999). However, there was a repeated complaint in European market about the honey from virgin intact forest areas, due to lower level of enzymes. Honey with low enzymatic level (diastase and invertase) and <15 mg HMF kg−1 can satisfy the quality standard (Fauzi and Farid 2015). Monofloral honeys found in Ethiopia and elsewhere are under frequent critics with respect to lower level of enzymes. These honeys are under investigation and not adequately researched so far. In order to describe the actual quality and merit of the honey, a compromise among the various quality control parameters of honey is necessary and may significantly benefit the poor rural forest dwellers engaged in forest beekeeping.
Various studies were under taken by a number of researchers on enzymes (Serrano et al. 2007; Sakač and Sak-Bosnar 2012; Lichtenberg-kraag 2014) amino acid profile (Hermosín et al. 2003; Iglesias et al. 2004) and HMF (Nässberger 1990; Arribas-Lorenzo and Morales 2010; Capuano and Fogliano 2011; Belay et al. 2013; Windsor et al. 2013) contents of honeys from different geographical region. However, honeys of Ethiopian origin from Acacia, Becium grandiflorum, Croton macrostachyus, Eucalyptus globulus, Hypoestes, Leucas abyssinica, Schefflera abyssinica, Syzygium guineense and Vernonia amygdalina; were not studied for their enzyme, amino acid and HMF levels. Thus, the purpose of this study was to investigate the enzyme levels, amino acid profiles and HMF composition of Ethiopian monofloral honeys.
Materials and methods
Sample collection and pollen analysis
Three hundred twenty honey samples were collected from Sheka (Masha and Andracha), Bonga (Chena and Gwata), Guji-Uraga, Illubabor (Becho and Yayu), Bale (Dello Mena and Angetu), Addis Ababa, Tigray (Wukro and Mayichew) and Amhara (Lalibella and Wag-Himra Ziqualla); Ethiopia. These areas are well known for their potential in honey production. Honey samples were collected from May 2014 to March 2015 based on their floral calendar. The 320 honey samples were categorized into nine monofloral honeys. From each sampling areas, a total of 80 honey samples were used for laboratory analysis. This helps to distinguish honeys of the same botanical origin vary in their value due to geographical position.
Beekeepers, at the farm gate, were selected using randomized lottery sampling methods. Late in the afternoon, the traditional beekeeper mounts on the tree using a long rope (about 50 m). The traditional hive was tightened using rope and transferred to the ground. The beekeeper puff smoke from the back (opposite to the entrance) and opens the hive. The vertically positioned fixed honey combs were clip from the top, brushed and put in dry plastic bucket. The honey combs were broken into pieces and strained using honey sieve, and allowed to settle in a 50 kg metallic honey container. These were done mostly in the South and South Western part of the country. Sample from Northern part of the country were from frame hives, which are extracted honey. Honey containing frame combs were clipped from the super box. The sealed frame combs were decapitated using uncapping fork and inserted into the honey extractor. Through centrifugation, the honey was drained from the cell and taken from the outlet of the honey extractor. Both the comb and extracted honeys was strained, settled, and later poured in 250 and 500 g food grade glass jar (Belay et al. 2015, 2017).
Pollen analysis was carried out to identify the dominant floras of the honey. It was determined through melissopalynology (Ohe et al. 2004; Ouchemoukh et al. 2007) and sorted into nine monofloral honeys, namely, Acacia, Becium grandiflorum, Croton macrostachyus, Eucalyptus globulus, Hypoestes, Leucas abyssinica, Schefflera abyssinica, Syzygium guineense and Vernonia amygdalina.
Enzyme analysis
Reagents and equipment
Phadebas tablets (Pharmacia Diagnostics Biologically Active Substances), sodium hydroxide (0.5 M), acetate buffer (0.1 M, pH 5.2), sodium acetate trihydrate, glacial acetic acid, potassium hydrogen phosphate (KH2PO4), disodium hydrogen phosphate (Na2HPO4·2H2O), p-nitrophenyl-α-d-glucopyranoside, pNPG (M Cat: 487506, Switzerland), tris-(hydroxymethyl) aminomethane, hydrochloric acid (HCl), spectrophotometer (Jenway, 6715UV/Vis), thermostated water bath (GFL Labortechnikmbh D3006 Burgwedel), vortex mixer (VWR Lab dancer), digital pH-meter (GS, TUV, Berlin), Whatman 1 filter paper (589/1, Whatman GmbH, Germany), 1 cm cuvettes (PMMA, Plastibrand, Cat. No. 7591 15), and reagent mixer (IKA Vortex 4 basic) were used for analysis of enzymes.
Diastase analysis
Diastase analysis was performed by Phadebas, based on Harmonized Method of the IHC using spectrophotometric method, in which an insoluble blue dyed starch hydrolyzed by the enzyme; yielding blue water-soluble fragments. 1 g of honey was weighed into a 100 mL volumetric flask, dissolved in the acetate buffer solution and filled to the mark. 5 mL of this solution was transferred to a test tube and placed in the water bath at 40 °C. Acetate buffer solution was prepared by dissolving 13.6 g of sodium acetate trihydrate in 1 L of distilled water, and the pH was adjusted to 5.2 by glacial acetic acid (1–2 mL). A blank was prepared by placing 5 mL aliquot of the acetate buffer in another test tube which is treated exactly as the sample solution. Phadebas tablet was added to both solutions using tweezers and the timer was started. Both solutions were stirred in the reagent mixer until the tablets disintegrated (ca. 10 s) and then returned to the water bath. The reaction was terminated by adding 1 mL sodium hydroxide solution, after exactly 15 min. The mixture stirred again in the reagent mixer for about 5 s. The solution was filtered through filter papers and poured into 1 cm cuvettes. The absorbance was measured using a spectrophotometer at 620 nm and distilled water was used as a reference. Diastase activity was computed by regression equation 28.2 × ΔA620 + 2.64; and ΔA620 was calculated by subtracting the absorbance of the blank from the sample solution (Bogdanov 2009).
Invertase analysis
Invertase levels of monofloral honeys were determined based on Harmonized Method of the IHC (Bogdanov 2009) with some modifications. 1 g of honey was dissolved in 10 mL buffer solution. The buffer solution (0.1 M; pH = 6.0) was prepared by dissolving 11.66 g of KH2PO4 and 2.56 g of Na2HPO4·2H2O in 1 L distilled water. At the same time 1.0 g of substrate solution, pNPG, was dissolved in 166 mL buffer solution. The reaction-terminating solution (3 M, pH = 9.5 adjust with 3 M HCl) was also prepared by dissolving 18.17 g tris-(hydroxymethyl) aminomethane in 50 mL buffer.
Substrate solution (1000 μL) was poured into a test tube and kept in the water bath (40 °C) for 5 min, before adding honey solution. 100 μL honey solution was added into the substrate and starts the reaction time. The content was briefly mixed using vortex and incubated at 40 °C for 20 min using thermostated water bath. After exactly 20 min, 100 μL of the reaction terminating solution was added and the solution was mixed again. For the blank, 1000 μL of substrate solution was incubated at 40 °C at the same time. After 5 min, 100 μL of reaction-terminating solution was added and mixed using vortex, and then 100 μL of honey solution was added. Blank was prepared for each honey sample tested. The solutions were kept at room temperature to cool, and the absorbance of the sample solutions and the blank were measured in 1 cm cell at 400 nm. The amount of p-nitrophenol, in μM, produced during the test corresponds exactly to the amount of substrate, in μM, utilized. Therefore, the honey invertase activity can be calculated from the absorbance measured at 400 nm and is indicated in units/kg (U kg−1):
where U = 1 international unit with a defined utilization of 1 μM per minute; ΔA400 = difference in absorbance between the honey sample and the blank.
Hydroxymethylfurfural analysis
HMF was determined in a clear, filtered, aqueous honey solution using reverse phase HPLC equipped with UV detector, adopted by Harmonized Method of the IHC. The signal was compared with those from standards of known concentration (Bogdanov 2009).
Reagents and equipment
5-(hydroxymethyl) furan-2-carbaldehyde, HMF (Flukar 53407, Lot no BCBM2548 VPCode101409210), water (HPLC grade, J.T. Baker, Avantor), methanol (HPLC grade, J.T. Baker, Avantor), Liquid chromatography with UV detector and integrator, Column: C18-reversed phase (4.6 mm i.d. × 250 mm, 5 μm pore size), membrane filter (0.45 μm, Cat No LTS022545, Agela Technologies), filter syringe (H 205 SW Henk Sass Wolf GmbH), and sample vials (Agilent, 8010-0014, USA) were used for HMF analysis.
Procedure
Five gram of honey sample was weighed into a 50 mL beaker and dissolved in 25 mL HPLC grade water. The solution was transferred into 50 mL volumetric flask, and filled to the mark with HPLC grade water. Then the solution was filtered through 0.45 μm membrane filter syringe and poured into sample vials for chromatography separation. HMF content of the sample was calculated by comparing the corresponding peak areas of the sample and those of the standard solutions, taking into account the dilution factor. The absorbance A of the prepared standard solution (1, 2, 5 and 10 g L−1) was determined using UV spectrophotometer at 285 nm in 1 cm quartz cells with water in the blank cell. A linear relationship between the concentration and peak area of HMF was observed with a regression coefficient (r2) of 0.992. Results were expressed in mg kg−1. HMF contents of honey samples were quantified using a regression equation, y = 57720x − 2584. Concentration (mg L−1) of the standard solutions was calculated using
where A is the absorbance of the standard solution.
HPLC condition
Analysis of honey samples for HMF content were conducted using Jasco (Tokyo, Japan) HPLC system with a PU-2089 Pulse gradient pump equipped with a degasser, AS-2075 Plus auto-sampler, and MD-2010 Plus diode array detector (DAD). Data was collected with the Jasco chrompass software. A comparative analysis was carried out using SunFire (Waters) C18 column (4.6 mm i.d. × 250 mm, 5 μm pore size). The following HPLC conditions were used throughout the measurement: flow rate of 1 mL min−1, column and detector temperature of 30 °C, sample volume of 40 µL, and UV detection at 285 nm. In addition, the mobile phase was a mixture of water and methanol (90:10, v/v). HPLC grade water and methanol were mixed and filtered using Whatman nylon 0.45 μm membrane filter (7404-004, G3620153, UK) and then sonicated (Branso Ultrasonics 5510E-DTH, USA).
Amino acid analysis
Reagents and equipment
Aspartic acid (Asp), Serine (Ser), Glutamic acid (Glu), Glycine (Gly), Histidine (His), Threonine (Thr), Arginine (Arg), Alanine (Ala), Proline (Pro), Tyrosine (Tyr), Valine (Val), Methionine (Met), Isoleucine (Ile), Lysine (Lys), Leucine (Leu) and Phenylalanine (Phe) (Sigma-Aldrich, USA), HCl (Junsei, Tokyo, Japan), Deionized water purified using Milli-Q System (Millipore, Bedford, MA, USA), Falcon polypropylene conical tube, digestion tube (Pyrex, Mexico), mixer (Thermo Scientific Vortex, M 37610-33, Malaysia), filter paper (No. 2, Advantec, Tokyo, Japan), vial (Agilent, USA), syringe filter (Agilent, USA), column (Ion exchange resin, 4.6 nm i.d., 60 mm length, 3 μm particle size, Tokyo Japan), and UV detector (Hitachi High-Technologies Corp.) were used for analysis of amino acids.
Procedure
Sample preparation, for analysis of amino acids, was performed based on Method 994.12 (AOAC 1990). Honey samples were soften and homogenized by immersing in a temperature controlled hot water (36 °C) using Falcon polypropylene conical tube. About 2 g of honey was weighed using digestion tube (Pyrex, Mexico) and 10 mL of 6 N HCl was added, and then mixed using a mixer (Thermo Scientific Vortex, 251 M 37610-33, Malaysia) thoroughly for 1 min. Nitrogen gas was purged into the digestion tube to remove air and then tightly capped. The digestion tube was placed in a dry oven at 110 °C for 22 h to hydrolyze the sample. After hydrolysis, the digestion tubes were allowed to cool in the dark at room temperature. The supernatant was transferred from the digestion tube into 50 mL volumetric flask and filled with distilled water up to the mark. The mixture was filtered through filter paper (No. 2, Advantec, Tokyo, Japan). 1 mL of filtered solution was transferred into 10 mL of volumetric flask and the solution was topped up to the mark with distilled water. About 0.8 mL of the solution was transferred into 2 mL vial (Agilent, USA) using 0.2 μm syringe filter (Agilent, USA), for injection and then subjected to chromatography separation.
For chromatographic separation, the analytical column used was Hitach HPLC Packed Column (Ion exchange resin, 4.6 mm i. d., 60 mm length, 3 μm particle size, Tokyo Japan) with Solfone (SO3 −) group as active exchange site. The analytical detector was visible detector (Hitachi High-Technologies Corp.) set to measure at 570 nm for all amino acid, except for proline 440 nm. Quantification of amino acid in monofloral honeys was performed based on the retention time of standard amino acid. The chromatogram of standard amino acids used as reference to honey sample is presented in Fig. 1.
Fig. 1.
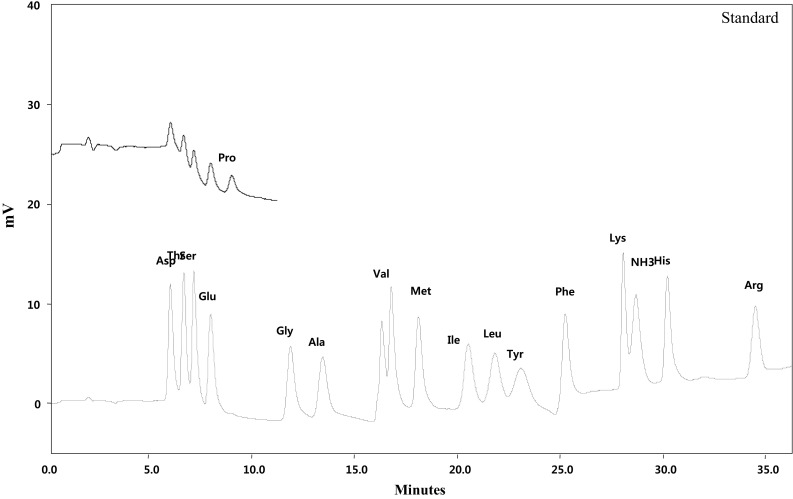
Chromatogram of standard amino acids: Asp (Aspartic acid), Thr (Threonine), Ser (Serine), Glu (Glutamic acid), Gly (Glycine), Ala (Alanine), Val (Valine), Methionine (Met), Ile (Isoleucine), Leu (Leucine), Tyr (Tyrosine), Phe (Phenylalanine), Lys (Lysine), NH3 (Ammonia), His (Histidine), Arg (Arginine) and Pro (Proline)
In this study, amino acid analysis of honey was done using amino acid analyzer (L-8900, Hitachi-High speed amino acid analyzer, Japan). The automated amino acid analyzer was validated using standards. The limits of detection and limits of quantification were less than 0.059 and 0.198 mg g−1, respectively (Shim et al. 2013).
Statistical analysis
Data for enzyme, amino acid and HMF were generated from multiple run of samples with a minimum of three replicate measurements. These data are presented in Table 1 as mean ± SD. Statistical analysis was carried using ANOVA, by SAS, 2002. Mean separation was computed using least square difference (LSD) at p < 0.01.
Table 1.
Mean ± SD for diastase, invertase and HMF value of Ethiopian monofloral honeys (n = 80)
| Monofloral | Diastase (Schade units) | Invertase (IN) | HMF (mg kg−1) |
|---|---|---|---|
| Acacia | 6.42 ± 0.690b | 14.5 ± 1.02e,f | 0.910 ± 0.370c,d,e |
| Becium grandiflorum | 5.84 ± 2.06b | 29.3 ± 4.53b | 0.480 ± 0.150e |
| Becium grandiflorum (L) | 13.5 ± 0.180a | ||
| Becium grandiflorum (W) | 11.6 ± 0.640e,f | ||
| Croton macrostachyus | 6.35 ± 0.570b | 13.6 ± 2.24e,f | 3.37 ± 1.73a |
| Eucalyptus globulus | 5.86 ± 0.890b | 20.7 ± 2.93d | 0.68 ± 0.050d,e |
| Hypoestes | 5.94 ± 1.37b,c | 15.2 ± 1.47e | 0 ± 0e |
| Leucas abyssinica | 6.20 ± 0.560b | 36.5 ± 1.93a | 0 ± 0e |
| Schefflera abyssinica | 4.94 ± 0.660c,d | 4.85 ± 2.36g | 1.13 ± 0.310d |
| Schefflera abyssinica (U) | 24.3 ± 6.56c | ||
| Syzygium guineense | 6.63 ± 0.340b | 19.7 ± 2.37d | 2.09 ± 0.210b,c |
| Vernonia amygdalina | 5.60 ± 1.54b,d | 11.6 ± 2.16f | 2.24 ± 0.560b |
Means in a column, for monofloral honey, with different letters were significantly different (p < 0.01)
IN: Invertase number; L: Lalibella; U: Uraga; W: Wukro
Results and discussions
Enzymes
Diastase activity
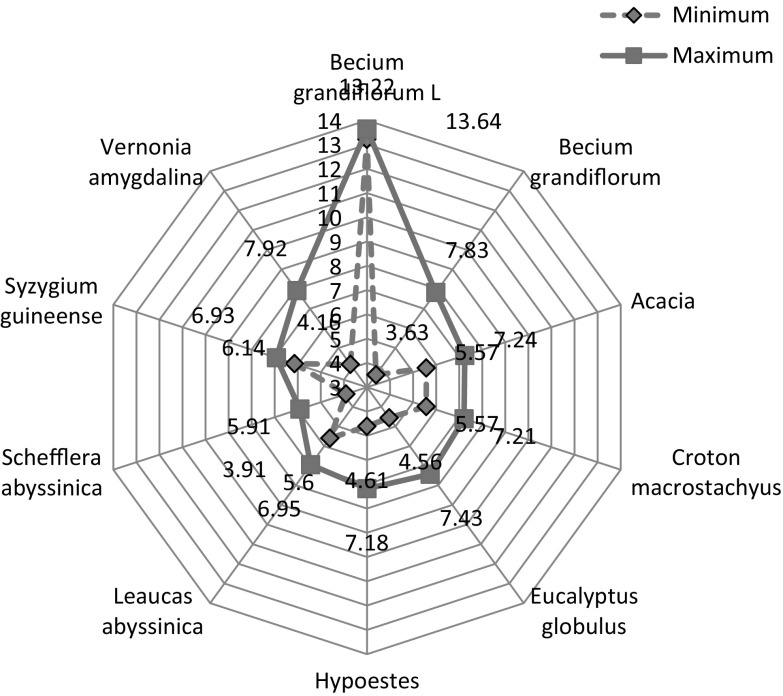
The spiders web (Fig. 2) showed the diastase activity of Ethiopian Honey. Diastase activity ranged from 3.91 (Schefflera abyssinica) to 13.6 (Becium grandiflorum). The mean and standard deviation of diastase and their significant differences of monofloral honeys are presented in Table 1. Becium grandiflorum (honey from Lalibella) showed the highest diastase activity. A significant difference (p < 0.01) was observed in diastase activity between Becium grandiflorum, honey from Lalibella, and all other honeys, including Becium grandiflorum honeys collected from Maychew and Wukro, Ethiopia. This indicated that honey from the same floral origin can possibly vary in enzyme activity, due to the contribution from the environment, in which the honey flora species grow and presence of different geographical races of bees, which is mainly governed by biotic and abiotic factors (Adgaba et al. 2017). According to Oddo et al. (1999) the enzyme content of honeys may differ based on the age of the bees that vary in race, the nectar gathering time, the physiological period of the colony, the quantity of nectar flow and its sugar content and pollen consumption.
Fig. 2.
Spider distribution of diastase
Becium grandiflorum, honey from Lalibella, was found to be the only honey within the CA and EU scale, ≥8 Schade scale (Bogdanov et al. 1999). All the other honey samples showed lower than eight Schade scale. This is in agreement with the study reported by Wang and Li (2011) that fresh honey, which did not expose to heat and from different plant origin, had lower diastase activity. Vansell and Freeborn (1929) quantified and described that certain comb honeys that could not have been heated are found to be too low in diastase to pass the official German test. Diastase is not only contributed from the nectar but also a result of glandular secretion of honeybees.
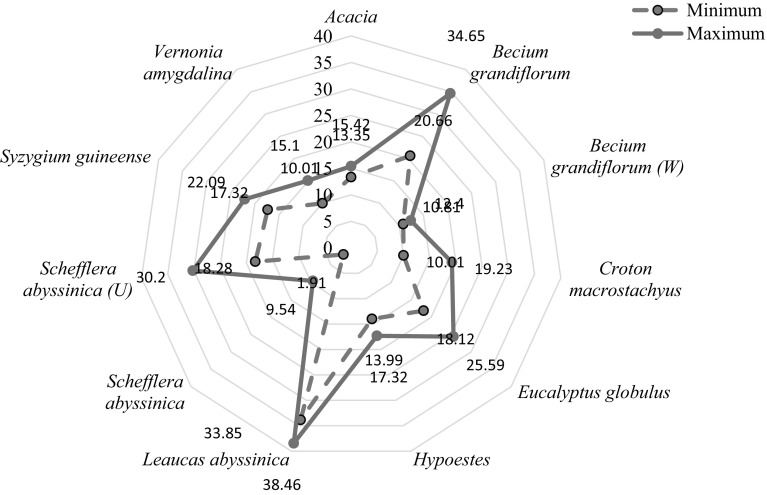
Invertase
The invertase distribution of Ethiopian honeys is presented in spiders web (Fig. 3). The figure showed that invertase (IN) activity ranged from 1.9 to 38.40. The mean and standard deviation of IN of monofloral honeys and their significant differences between treatments are presented in Table 1. Treatments of monofoloral honeys were set based on their floral origin. Based on invertase classification of honey (Oddo et al. 1999), the Ethiopian monofloral honeys were found in all classes: low IN < 10 (Schefflera abyssinica); medium to high IN 10–20 (Acacia, Becium grandiflorum (W), Hypoestes and Croton macrostachyus) and high IN > 20 (Becium grandiflorum, Vernonia amygdalina, Eucalyptus globulus, Leucas abyssinica, Schefflera abyssinica (U) and Syzygium guineense). Leucas abyssinica has the highest IN (36.5 ± 1.93) and Schefflera abyssinica (except for Schefflera abyssinica from Uraga) had the lowest. The reason for the variability in invertase activity of Ethiopian honeys could be due to the difference in their geographical origin. A significant difference (p < 0.01) was observed in invertase activity between Leucas abyssinica and the other monofloral honeys. Honeys of the same botanical origin from Schefflera abyssinica and Becium grandiflorum showed a significant difference (p < 0.01) in IN, regardless of their botanical origin. Hence, geographical races of bees have an effect on invertase content of Ethiopian honeys.
Fig. 3.
Spider distribution of invertase
HMF content
HMF content of Ethiopian honeys ranged from 0 (Hypoestes and Leucas abyssinica) to 3.37 ± 1.73 (Croton macrostachyus). The mean and standard deviation of HMF content of Ethiopian honey is presented in Table 1. Hypoestes and Leucas abyssinica were found to be significantly different (p < 0.01) in HMF content compared to Croton macrostachyus, Schefflera abyssinica, Syzygium guineense and Vernonia amygdalina honeys.
IHC and regional standards widely consider HMF as a marker of quality deterioration. In addition, Codex Alimentarius (Alinorm 01/25 2000) declared that the HMF content of honey must not be higher than 80 mg kg−1. The European Union (EU Directive 110/2001) also stated that the HMF content of honey to be 40 mg kg−1 with the exceptions that: 80 mg kg−1 for honey from Countries or Regions with tropical temperatures, and 15 mg kg−1 for honey with low enzymatic level (8–3 Schade units) (Zappala et al. 2005). Excessive heating or inappropriate storage conditions of honey can cause the production of undesirable compounds and resulted in loss of honey quality (Truzzi et al. 2012). Singh and Bath (1997) also reported that with increasing heating time of 0–30 min, an increased in intensity of HMF formation for Trifolium, E. lanceolatus and B. juncea honeys (Indian honeys) at 65 °C. Hence, the concern of HMF level in honey is related to toxicity. HMF compound and its derivatives (5-chloromethyl and 5-sulfidemethylfurfural) have been shown to have genotoxic (Severin et al. 2010), mutagenic and carcinogenic (Durling et al. 2009) effects. All the Ethiopian monofloral honeys satisfied the maximum acceptable limit of CA, EU, Ethiopian standards, the international trade and the sever limits of the European bee federation.
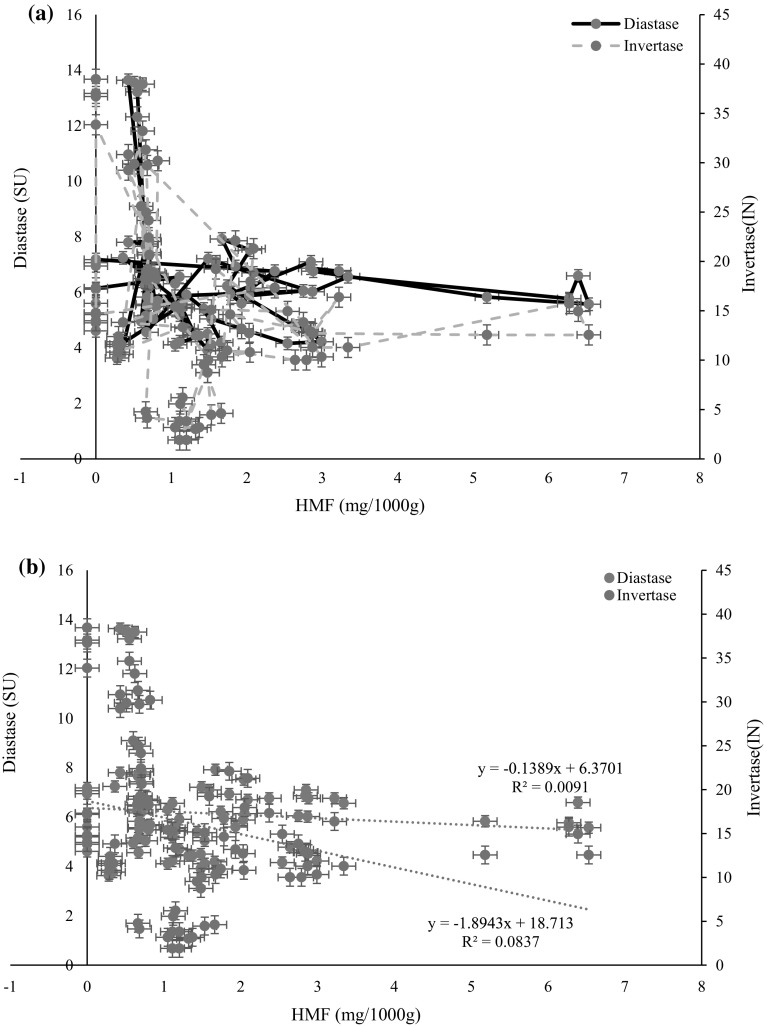
Regression analysis of diastase and invertase to HMF
Regression analysis was performed between diastase and HMF; and invertase and HMF. The aldehyde 5-hydroxymethylfurfural (HMF) is formed as a result of sugar degradation via the Maillard reaction and caramelization (Turhan et al. 2008). The development of HMF in honey can be considered as an important freshness and quality indicator, because elevated concentration is a sign for overheating, long time storage in poor conditions or aging of the honey. International honey standards authorities repeatedly used HMF as a reliable quality parameters. Enzymes are also used as a quality parameter; even though, some honey do have a lower level of enzymes intrinsically (Bogdanov et al. 1999; White 1994). In line with this, HMF level of honey is used to verify, whether the lower level of enzyme is intrinsic or extrinsic characteristic of the honey. The use of HMF and enzyme activity for honey quality determination is firmly rooted in honey quality legislation, and illustrated by the honey standard authorities of the Codex Alimentarius and European Union (White 1994). According to Zappala et al. (2005) and White (1994) honeys with lower level of enzyme, needs to consist essentially a maximum of 15 mg kg−1 of HMF to satisfy international standards. This help to prove that honey has not undergone heat treatment or prolonged storage. In this study, regression analysis was performed between diastase and HMF; and invertase and HMF (Fig. 4). Regression equations for diastase to HMF, and invertase to HMF were y = −0.1389x + 6.3701 and y = −1.8943x + 18.713, respectively. The results of the regression analysis indicated that both diastase and invertase enzymes found negatively regress with HMF.
Fig. 4.
Scattered plot of a diastase and invertase values to HMF content (markers joined by straight lines); b linear regression of diastase and invertase to HMF
The regression graph diastase and invertase to HMF for nine honey samples is presented in Fig. 4. Regression equations for diastase to HMF, and invertase to HMF were y = −0.1389x + 6.3701 and y = −1.8943x + 18.713, respectively. The results of the regression analysis indicated that both diastase and invertase enzymes showed significant (p < 0.05) negative relation with HMF.
Amino acids composition
The mean concentrations and standard deviations of amino acids for monofloral honeys: Acacia, Becium grandiflorum, Croton macrostachyus, Eucalyptus globulus, Hypoestes, Leucas abyssinica and Schefflera abyssinica; are presented in Table 2. Three amino acids (phenylalanine, proline and aspartic acid) were found in higher concentrations. The contents of proline and phenylalanine in Eucalyptus globulus honey were found to be 47.6 ± 1.87 and 8.6 ± 0.26 mg/100 g, respectively (Table 2). This is in agreement with study reported by Hermosín et al. (2003) for Eucalyptus globulus honey with mean value of 49.3 and 10.7 mg/100 g for proline and phenylalanine, respectively. A number of researchers (Iglesias et al. (2004) and Truzzi et al. (2014) also indicated that proline is the predominant amino acid fraction in honey. This is in line with the results of some Ethiopian monofloral honeys, namely, Croton macrostachyus, Eucalyptus globulus and Schefflera abyssinica (Iglesias et al. 2004; Truzzi et al. 2014). In contrast, honey from Acacia, Becium grandiflorum, Hypoestes, and Leucas abyssinica were found to be high in phenylalanine than proline. Tyrosine was found in Acacia honey (9.90 mg/100 g), but not detected in other monofloral honeys. The analysis of variance model results showed that there was a significant difference between monofloral honeys in their content of specific amino acid (Table 2).
Table 2.
Amino acids content (mean ± SD) of monofloral honey (mg/100 g)
| Variable | Acacia | Becium grandiflorum | Croton macrostachyus | Eucalyptus globulus | Hypoestes | Leucas abyssinica | Schefflera abyssinica |
|---|---|---|---|---|---|---|---|
| Aspartic acid | 22.0 ± 0.300a | 9.74 ± 0.0400c | 21.9 ± 0.690a | 12.8 ± 0.640b | 8.84 ± 0.00cd | 11.9 ± 0.190b | 8.15 ± 0.290d |
| Threonine | 9.40 ± 0.0210a | 3.13 ± 0.054ef | 6.37 ± 0.300b | 4.28 ± 0.150c | 3.19 ± 0.050e | 3.84 ± 0.170d | 2.79 ± 0.130f |
| Serine | 9.46 ± 0.010a | 4.15 ± 0.00d | 9.54 ± 0.350a | 6.41 ± 0.240b | 3.92 ± 0.040d | 5.00 ± 0.07c | 3.25 ± 0.00e |
| Glutamic acid | 24.8 ± 0.160a | 7.24 ± 0.250d | 23.0 ± 0.220b | 10.1 ± 0.360c | 6.22 ± 0.250e | 9.99 ± 0.300c | 5.03 ± 0.020f |
| Proline | 74.1 ± 2.34a | 23.8 ± 0.700e | 53.3 ± 0.370b | 47.6 ± 1.87c | 25.2 ± 0.300e | 33.1 ± 0.680d | 15.5 ± 0.930f |
| Glycine | 7.72 ± 0.360b | 2.02 ± 0.00d | 11.2 ± 0.400a | 2.99 ± 0.050c | 1.84 ± 0.050d | 2.72 ± 0.040c | 1.69 ± 0.020d |
| Alanine | 7.18 ± 0.230b | 2.43 ± 0.080d | 7.74 ± 0.150a | 3.89 ± 0.130c | 2.61 ± 0.090d | 3.91 ± 0.050c | 1.96 ± 0.080e |
| Valine | 9.12 ± 0.390a | 2.12 ± 0.060d | 4.98 ± 0.130b | 2.47 ± 0.089d | 1.70 ± 0.046e | 2.95 ± 0.170c | 1.58 ± 0.040e |
| Methionine | 0.88 ± 0.030b | 0.65 ± 0.030d | 1.11 ± 0.01a | 0.76 ± 0.044c | 0.47 ± 0.00e | 0.84 ± 0.04bc | 0.22 ± 0.00f |
| Isoleucine | 5.95 ± 0.380a | 2.40 ± 0.090d | 4.10 ± 0.110b | 2.54 ± 0.080d | 2.75 ± 0.050d | 3.45 ± 0.190c | 1.57 ± 0.080e |
| Leucine | 10.1 ± 0.450a | 4.97 ± 0.040c | 6.27 ± 0.10b | 4.14 ± 060.07d | 2.87 ± 0.06e | 4.46 ± 0.05d | 2.53 ± 0.040e |
| Tyrosine | 9.90 ± 0.390a | 0.00 ± 0.00b | 0.00 ± 0.00b | 0.00 ± 0.00b | 0.00 ± 0.00b | 0.00 ± 0.00b | 0.00 ± 0.00b |
| Phenylalanine | 118.9 ± 0.910a | 61.4 ± 1.64b | 31.62 ± 0.940c | 8.62 ± 0.260d | 63.1 ± 4.09b | 61.1 ± 1.05b | 5.09 ± 0.090d |
| Lysine | 4.98 ± 0.180b | 3.33 ± 0.0420e | 7.09 ± 0.280a | 3.89 ± 0.110d | 3.16 ± 0.110e | 4.39 ± 0.130c | 2.88 ± 0.010f |
| Histidine | 4.54 ± 0.080a | 1.06 ± 0.020d | 2.89 ± 0.080b | 1.87 ± 0.050c | 1.17 ± 0.040d | 1.87 ± 0.050c | 1.01 ± 0.010e |
| Arginine | 1.34 ± 0.010c | 1.12 ± 0.042d | 2.69 ± 0.160a | 1.61 ± 0.060b | 0.93 ± 0.030e | 1.14 ± 0.060d | 1.06 ± 0.020de |
Means in a raw, for monofloral honey, with different letters were significantly different (p < 0.01)
Amino acid level, especially proline, is used as indicator for honey ripeness and in some cases sugar adulteration. Even though there was a significant variation between floral types, a minimum limit of 180 mg proline kg−1 honey has been accepted as a quality standard in honey control laboratories (Bogdanov et al. 1999). Based on this criterion, all the Ethiopian monofloral honeys had a value above the minimum acceptable limit.
Conclusion
Honeys from Becium grandiflorum and Schefflera abyssinica were found to be significantly different in diastase activity and invertase number, regardless of their botanical origin. Even though the honey samples are fresh, ripened and from the comb; most of the honeys contain lower amount of enzymes. The lower levels of enzymes, diastase and invertase could be intrinsic characteristics of Ethiopian monofloral honeys. Three amino acids phenylalanine, proline and aspartic acid were found in relatively higher concentrations than other amino acids. The findings of this study indicated that the enzyme level of monofloral honeys could be affected by geographical position, which is mostly related to the race of bees. Thus, the enzyme level alone cannot be a worthwhile indicator for the quality control, unless it is supported by other quality control parameters, mainly HMF. Further investigation is recommended on secretion capability of hyphopharengeal gland of honeybees of different geographical races, and the contributions of honeybees and floral origin to diastase activity and invertase number.
Acknowledgements
We would like to thank SNV Ethiopia, GIZ-SLM, MELCA Ethiopia for their logistical support; Korean Food Research Institute (KFRI), Fulda University of Applied Sciences, Germany, for laboratory service; Phadebas for providing reagents for enzyme analysis; Dr. Jurgen Greiling, Prof. Mooha Lee for coordinating the field work and laboratories abroad; and Hirut Abebe and Abate Geremew for their assistance in the lab.
Contributor Information
Abera Belay, Email: ab.berabelay@gmail.com.
Gulelat Desse Haki, Email: gulelatw@yahoo.com.
Marc Birringer, Email: Marc.Birringer@he.hs-fulda.de.
Hannelore Borck, Email: hannelore.borck@he.hs-fulda.de.
Young-Chul Lee, Email: barumin@hanmail.net.
Kyung-Tack Kim, Email: tack@kfri.re.kr.
Kaleab Baye, Email: kaleabbaye@gmail.com.
Samuel Melaku, Phone: +0017065078476, Email: abegaz_samuel@columbusstate.edu, Email: samuelmelaku@yahoo.com.
References
- Adgaba N, Al-Ghamdi A, Tadesse Y, Getachew A, Awad AM, Ansari MJ, Alqarni AS. Nectar secretion dynamics and honey production potentials of some major honey plants in Saudi Arabia. Saudi J Biol Sci. 2017;24:180–191. doi: 10.1016/j.sjbs.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arribas-Lorenzo G, Morales FJ. Estimation of dietary intake of 5-hydroxymethylfurfural and related substances from coffee to Spanish population. Food Chem Toxicol. 2010;48(2):644–649. doi: 10.1016/j.fct.2009.11.046. [DOI] [PubMed] [Google Scholar]
- Association of Official Analytical Chemists (AOAC) Official methods of the analysis of official analytical chemists. 15. Virginia: AOAC; 1990. [Google Scholar]
- Belay A, Solomon WK, Bultossa G, Adgaba N, Melaku S. Physicochemical properties of the Harenna forest honey, Bale, Ethiopia. Food Chem. 2013;141(4):3386–3392. doi: 10.1016/j.foodchem.2013.06.035. [DOI] [PubMed] [Google Scholar]
- Belay A, Solomon WK, Bultossa G, Adgaba N, Melaku S. Botanical origin, colour, granulation, and sensory properties of the Harenna forest honey, Bale, Ethiopia. Food Chem. 2015;167:213–219. doi: 10.1016/j.foodchem.2014.06.080. [DOI] [PubMed] [Google Scholar]
- Belay A, Haki GD, Birringer M, Borck H, Addi A, Baye K, Melaku S. Rheology and botanical origin of Ethiopian monofloral honey. LWT-Food Sci Technol. 2017;75:393–401. doi: 10.1016/j.lwt.2016.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanov S. Harmonised methods of the international. IHC. 2009;5:1–62. [Google Scholar]
- Bogdanov S, Lüllmann C, Martin P, von der Ohe W, Russmann H, Vorwohl G, Flamini C. Honey quality and international regulatory standards: review by the International Honey Commission. Bee World. 1999;80(2):61–69. doi: 10.1080/0005772X.1999.11099428. [DOI] [Google Scholar]
- Capuano E, Fogliano V. Acrylamide and 5-hydroxymethylfurfural (HMF): a review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT-Food Sci Technol. 2011;44(4):793–810. doi: 10.1016/j.lwt.2010.11.002. [DOI] [Google Scholar]
- De Smet L, Saegerman C, Ravoet J, de Graaf DC. Hydroxylmethylfurfural induces reactive oxygen species (ROS)-dependent activation of the Toll pathway in honey bees. Julius-Kühn-Archiv. 2015;450:296. [Google Scholar]
- Durling LJ, Busk L, Hellman BE. Evaluation of the DNA damaging effect of the heat-induced food toxicant 5-hydroxymethylfurfural (HMF) in various cell lines with different activities of sulfotransferases. Food Chem Toxicol. 2009;47(4):880–884. doi: 10.1016/j.fct.2009.01.022. [DOI] [PubMed] [Google Scholar]
- Fallico B, Zappala M, Arena E, Verzera A. Effects of conditioning on HMF content in unifloral honeys. Food Chem. 2004;85(2):305–313. doi: 10.1016/j.foodchem.2003.07.010. [DOI] [Google Scholar]
- Fauzi NA, Farid MM. High-pressure processing of Manuka honey: brown pigment formation, improvement of antibacterial activity and hydroxymethylfurfural content. Int J Food Sci Technol. 2015;50(1):178–185. doi: 10.1111/ijfs.12630. [DOI] [Google Scholar]
- Ferreira IC, Aires E, Barreira JC, Estevinho LM. Antioxidant activity of Portuguese honey samples: different contributions of the entire honey and phenolic extract. Food Chem. 2009;114(4):1438–1443. doi: 10.1016/j.foodchem.2008.11.028. [DOI] [Google Scholar]
- Gürkan R, Altunay N. Quantification of 5-hydroxymethylfurfural in honey samples and acidic beverages using spectrophotometry coupled with ultrasonic-assisted cloud point extraction. J Food Compos Anal. 2015;42:141–151. doi: 10.1016/j.jfca.2015.03.012. [DOI] [Google Scholar]
- Hermosín I, Chicón RM, Cabezudo MD. Free amino acid composition and botanical origin of honey. Food Chem. 2003;83(2):263–268. doi: 10.1016/S0308-8146(03)00089-X. [DOI] [Google Scholar]
- Iglesias MT, de Lorenzo C, Polo MDC, Martín-Álvarez PJ, Pueyo E. Usefulness of amino acid composition to discriminate between honeydew and floral honeys. Application to honeys from a small geographic area. J Agric Food Chem. 2004;52(1):84–89. doi: 10.1021/jf030454q. [DOI] [PubMed] [Google Scholar]
- Jasicka-Misiak I, Poliwoda A, Dereń M, Kafarski P. Phenolic compounds and abscisic acid as potential markers for the floral origin of two Polish unifloral honeys. Food Chem. 2012;131(4):1149–1156. doi: 10.1016/j.foodchem.2011.09.083. [DOI] [Google Scholar]
- Karabournioti S, Zervalaki P. The effect of heating on honey HMF and invertase. Apiacta. 2001;36(4):177–181. [Google Scholar]
- Lazarević KB, Andrić F, Trifković J, Tešić Ž, Milojković-Opsenica D. Characterisation of Serbian unifloral honeys according to their physicochemical parameters. Food Chem. 2012;132(4):2060–2064. doi: 10.1016/j.foodchem.2011.12.048. [DOI] [Google Scholar]
- Lichtenberg-Kraag B. Evidence for correlation between invertase activity and sucrose content during the ripening process of honey. J Apic Res. 2014;53(3):364–373. doi: 10.3896/IBRA.1.53.3.03. [DOI] [Google Scholar]
- Nässberger L. Influence of 5-hydroxymethylfurfural (5-HMF) on the overall metabolism of human blood cells. Hum Exp Toxicol. 1990;9(4):211–214. doi: 10.1177/096032719000900402. [DOI] [PubMed] [Google Scholar]
- Oddo LP, Piazza MG, Pulcini P. Invertase activity in honey. Apidologie. 1999;30(1):57–65. doi: 10.1051/apido:19990107. [DOI] [Google Scholar]
- Ohashi K, Natori S, Kubo T. Expression of amylase and glucose oxidase in the hypopharyngeal gland with an age-dependent role change of the worker honeybee (Apis mellifera L.). Euro. J Biochem. 1999;265(1):127–133. doi: 10.1046/j.1432-1327.1999.00696.x. [DOI] [PubMed] [Google Scholar]
- Ohe WVD, Oddo LP, Piana ML, Morlot M, Martin P. Harmonized methods of melissopalynology. Apidologie. 2004;35:S18–S25. doi: 10.1051/apido:2004050. [DOI] [Google Scholar]
- Ouchemoukh S, Louaileche H, Schweitzer P. Physicochemical characteristics and pollen spectrum of some Algerian honeys. Food Control. 2007;18(1):52–58. doi: 10.1016/j.foodcont.2005.08.007. [DOI] [Google Scholar]
- Paramás AMG, Bárez JAG, Marcos CC, García-Villanova RJ, Sánchez JS. HPLC-fluorimetric method for analysis of amino acids in products of the hive (honey and bee-pollen) Food Chem. 2006;95(1):148–156. doi: 10.1016/j.foodchem.2005.02.008. [DOI] [Google Scholar]
- Sakač N, Sak-Bosnar M. A rapid method for the determination of honey diastase activity. Talanta. 2012;93:135–138. doi: 10.1016/j.talanta.2012.01.063. [DOI] [PubMed] [Google Scholar]
- Serrano S, Espejo R, Villarejo M, Jodral ML. Diastase and invertase activities in Andalusian honeys. Int J Food Sci Technol. 2007;42(1):76–79. doi: 10.1111/j.1365-2621.2006.01213.x. [DOI] [Google Scholar]
- Severin I, Dumont C, Jondeau-Cabaton A, Graillot V, Chagnon MC. Genotoxic activities of the food contaminant 5-hydroxymethylfurfural using different in vitro bioassays. Toxicol Lett. 2010;192(2):189–194. doi: 10.1016/j.toxlet.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Shim YS, Yoon WJ, Ha J, Seo D, Lee KW, Lee WY, Kwak HJ. Method validation of 16 types of structural amino acids using an automated amino acid analyzer. Food Sci Biotech. 2013;22(6):1567–1571. doi: 10.1007/s10068-013-0252-0. [DOI] [Google Scholar]
- Singh N, Bath PK. Quality evaluation of different types of Indian Honey. Food Chem. 1997;58(1–2):129–133. doi: 10.1016/S0308-8146(96)00231-2. [DOI] [Google Scholar]
- Truzzi C, Annibaldi A, Illuminati S, Finale C, Rossetti M, Scarponi G. Determination of very low levels of 5-(hydroxymethyl)-2-furaldehyde (HMF) in Natural Honey: comparison between the HPLC technique and the spectrophotometric white method. J Food Sci. 2012;77(7):C784–C790. doi: 10.1111/j.1750-3841.2012.02782.x. [DOI] [PubMed] [Google Scholar]
- Truzzi C, Annibaldi A, Illuminati S, Finale C, Scarponi G. Determination of proline in honey: comparison between official methods, optimization and validation of the analytical methodology. Food Chem. 2014;150:477–481. doi: 10.1016/j.foodchem.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Turhan I, Tetik N, Karhan M, Gurel F, Tavukcuoglu HR. Quality of honeys influenced by thermal treatment. LWT-Food Sci Technol. 2008;41(8):1396–1399. doi: 10.1016/j.lwt.2007.09.008. [DOI] [Google Scholar]
- Vansell GH, Freeborn SB. Preliminary report on the investigations of the source of diastase in honey. J Econ Entomol. 1929;22(6):922–926. doi: 10.1093/jee/22.6.922. [DOI] [Google Scholar]
- Wang J, Li QX. Chemical composition, characterization, and differentiation of honey botanical and geographical origins. Adv Food Nutr Res. 2011;62:89–137. doi: 10.1016/B978-0-12-385989-1.00003-X. [DOI] [PubMed] [Google Scholar]
- White JW. The role of HMF and diastase assays in honey quality evaluation. Bee World. 1994;75(3):104–117. doi: 10.1080/0005772X.1994.11099213. [DOI] [Google Scholar]
- Windsor S, Kavazos K, Brooks P. The quantitation of hydroxymethylfurfural in Australian Leptospermum honeys. J Pharmacogn Phytother. 2013;5(1):21–25. [Google Scholar]
- Zappala M, Fallico B, Arena E, Verzera A. Methods for the determination of HMF in honey: a comparison. Food Control. 2005;16(3):273–277. doi: 10.1016/j.foodcont.2004.03.006. [DOI] [Google Scholar]