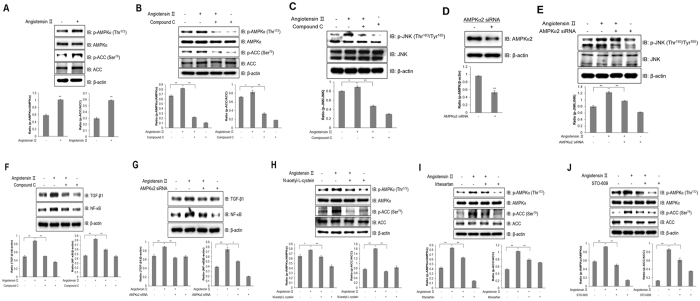
Figure 6.
Ang ІІ activates inflammation through AMPK in HL-1 atrial cells. (A) HL-1 cells were stimulated with 1 μM Ang ІІ for 24 h. Cell lysates were analysed by western blotting with antibodies against phosphorylated AMPKα2 and ACC. Levels of total AMPK and ACC were used as controls, and β-actin served as a protein loading control. (B) HL-1 cells were pre-treated with compound C (5 μM) for 30 min and treated with Ang ІІ for 24 h. AMPKα and ACC phosphorylation was quantified by western blot analysis using antibodies specific to the phosphorylated proteins. Total AMPK, ACC, and β-actin levels were measured as controls for protein loading. (C) Cells were stimulated with Ang ІІ for 24 h. Proteins were immunoprecipitated with the AMPKα antibody. Protein expression was analysed via western blot analysis by using JNK antibody. (D) HL-1 cells were stimulated with Ang ІІ in the presence of compound C. The protein expression of phosphorylated JNK, total JNK, and β-actin was analysed by western blotting. Blotting with antibodies specific to JNK and β-actin served as controls. (E) HL-1 cells were transiently transfected with AMPKα2 siRNA (100 nM) for 2 days and then treated with Ang ІІ (1 μM) for 24 h. Cell lysates were assessed by western blotting with AMPK and β-actin antibodies. (F) AMPKα2 siRNA was transiently transfected into cells followed by Ang ІІ treatment. Cells were lysed, and p-JNK, JNK, and β-actin expression levels were quantified by western blotting. JNK and β-actin levels were also measured as protein loading controls. (G) HL-1 cells were pre-treated with compound C for 30 min and treated with Ang ІІ for 24 h. TGF-β1 and NF-κB expression levels were quantified by western blot analysis using antibodies specific to the phosphorylated proteins. β-actin level was measured as a protein loading control. (H) HL-1 cells were transiently transfected with AMPKα2 siRNA and treated with Ang ІІ. Cell lysates and TGF-β1, NF-κB, and β-actin levels were analysed by western blotting. (I) HL-1 cells were treated with Ang ІІ in the presence of NAC. Protein expression of phosphorylated AMPKα and ACC was measured by immunoblotting with specific antibodies to the phosphorylated proteins. Total AMPK, ACC, and β-actin levels were quantified as controls. (J) HL-1 cells were stimulated with Ang ІІ in the presence of irbesartan. AMPKα and ACC phosphorylation was assessed by western blotting using specific antibodies, and total AMPKα, ACC, and β-actin levels were quantified as controls. (K) HL-1 cells were pre-treated with STO-609 and then treated with Ang ІІ. Cell lysates were analysed by western blotting with antibodies against phosphorylated AMPKα and ACC. Non-phosphorylated AMPKα and ACC were used as controls. β-actin served as a protein loading control. *P < 0.05, **P < 0.01, compared with the untreated cells. Results are from three independent experiments. Blots are displayed in cropped format.