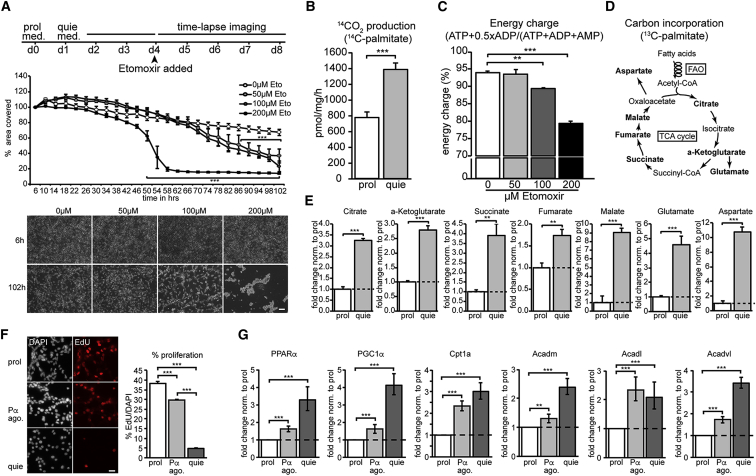
Figure 2.
High Levels of FAO Are Required to Sustain Cellular Quiescence
(A) Time-lapse analysis of quiescent NSPCs exposed to various doses of the irreversible Cpt1 inhibitor Etomoxir (50, 100, and 200 μM). Shown are a schematic outline of the experimental setup, the quantification of the area covered by quiescent NSPCs over time, and representative images. Complete block of FAO by Etomoxir during quiescence leads to a dramatic and dose-dependent decrease of area covered over time, caused by cell death upon FAO inhibition (mean ± SEM).
(B–E) Quiescent NSPCs use FAO for energy purposes and may use it as an alternative carbon source.
(B) Radioactive FAO measurements using 14C-labeled palmitic acid revealed a significant increase in 14CO2 in quiescent NSPCs compared to proliferating NSPCs, suggesting that at least part of the fatty acids are fully oxidized and might be used for energy purposes (mean ± SD).
(C) Energy charge measurements in quiescent NSPCs show that FAO indeed contributes to the amount of ATP generated, as treatment with various doses of Etomoxir (50, 100, and 200 μM) reduced the energy charge significantly (mean ± SEM).
(D) Scheme outlining the path of 13C-labeled palmitic acid upon FAO. The oxidation of fatty acids results in acetyl-CoA, which can be fed into the TCA. The metabolites measured with mass spectrometry in a 13C-incorporation assay are shown in bold.
(E) Quiescent NSPCs show an increase in 13C-incorporation in TCA intermediates and amino acids derived from TCA intermediates compared to proliferating NSPCs (mean ± SD). These 13C-incorporation assay results suggest that fatty acids might also serve as an alternative carbon source in quiescent NSPCs.
(F) High levels of FAO in quiescent NSPCs are at least partially regulated through the transcription factor PPARα. Treatment for 48 hr with 100 μM of the PPARα agonist WY14643 in proliferating NSPCs significantly reduced proliferation compared to control NSPCs, but proliferation was still far higher than in quiescent NSPCs, as assessed by EdU pulsing. Shown are representative images and the corresponding quantification of EdU-positive cells (mean ± SEM).
(G) WY14643 treatment led to an upregulation of PPARα and its target FAO genes in proliferating NSPCs compared to control NSPCs, however, to a far lesser extent than in quiescent NSPCs. Shown are the mRNA expression levels (mean fold change ± range) of PPARα, Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α), Cpt1a, medium-chain acyl-CoA dehydrogenase (Acadm), long-chain acyl-CoA dehydrogenase (Acadl), and very long-chain acyl-CoA dehydrogenase (Acadvl).
Scale bar represents 50 μm (A) and 20 μm (F). ∗∗∗p < 0.001; ∗∗p < 0.01.