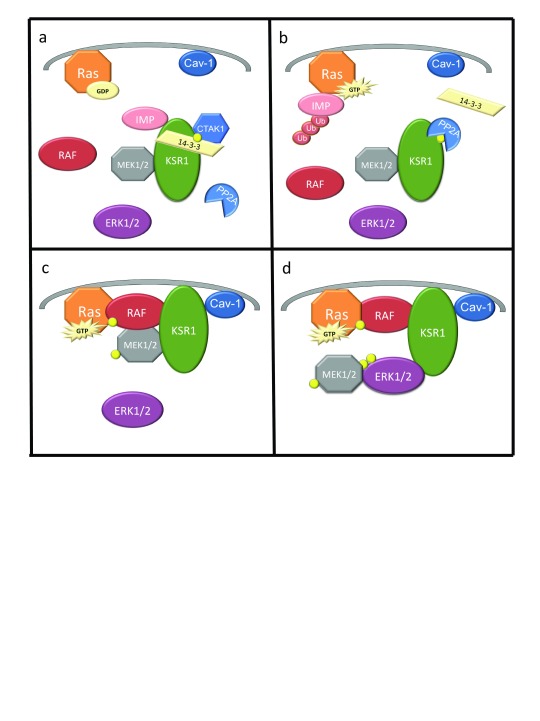
Figure 1. KSR1 dynamically regulates the Raf/MEK/ERK kinase cascade.
( a) KSR1 is constitutively bound to MEK1/2 and IMP. C-TAK1 phosphorylates (yellow circle) KSR1 at Ser392, allowing for 14-3-3 binding and cytoplasmic localization of KSR1. ( b) Upon Ras activation and GTP binding, IMP dissociates from KSR1, binds Ras, autoubiquitylates, and is degraded. PP2A dephosphorylates KSR1 at Ser392, destroying the 14-3-3 binding site anchoring KSR1. ( c) KSR1 and MEK1/2 translocate to the plasma membrane, where KSR1 interacts with Raf and MEK1/2 is phosphorylated and activated. ( d) MEK1/2 dissociates from KSR1 and ERK1/2 is phosphorylated and associates with KSR1, facilitating signaling. Cav-1, caveolin-1; C-TAK1, Cdc25C-associated kinase 1; ERK, extracellular signal-regulated protein kinase; GTP, guanosine triphosphate; IMP, impedes mitogenic signal propagation; KSR1, Kinase Suppressor of Ras 1; MEK, mitogen-activated protein/extracellular signal-regulated protein kinase kinase; PP2A, protein phosphatase 2.