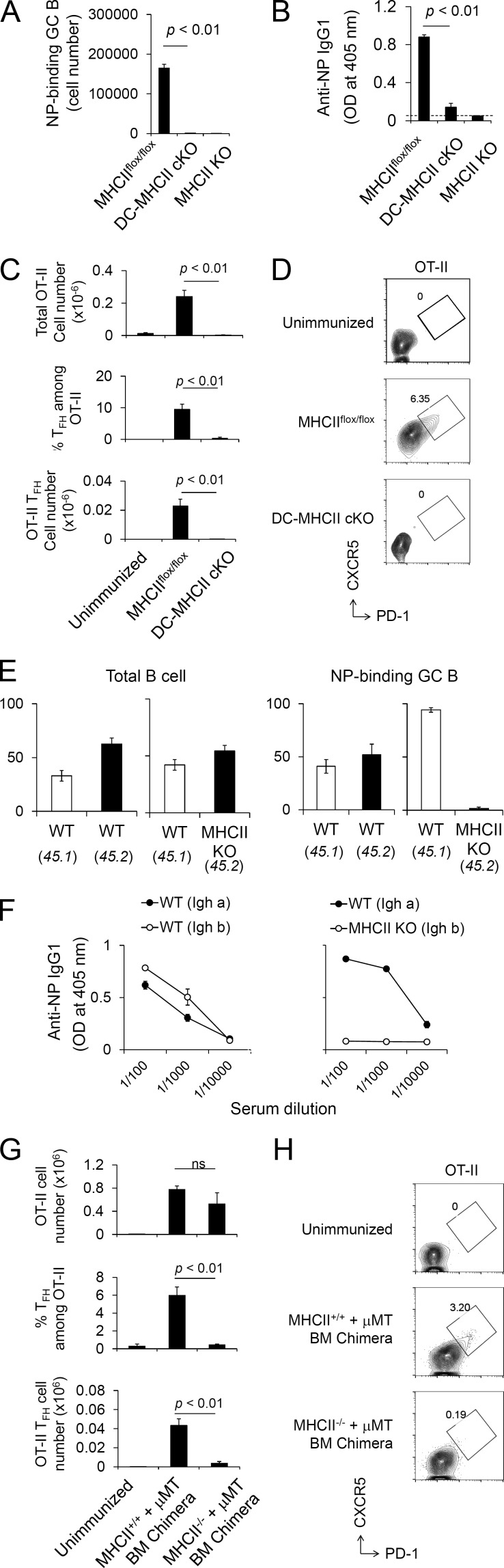
Figure 1.
Nonredundant requirements for MHCII on DCs and B cells in GC response. (A) DC-specific MHCII cKO (MHCIIflox/flox × CD11c-Cre, termed DC-MHCII cKO) mice were immunized with NP-KLH/Alum. At day 7 after immunization, NP-specific GC B cell numbers were analyzed in the spleen. Each strain, n = 3. Data are representative of three independent experiments. (B) DC-MHCII cKO mice were immunized with NP-KLH/Alum. 3 wk after immunization, anti-NP IgG1 titers were analyzed by ELISA. Dashed line indicates background OD value in empty wells. MHCIIflox/flox, n = 4; DC-MHCII cKO, n = 4; and MHCII KO, n = 2. Data are representative of two independent experiments. (C and D) OT-II T cells (CD45.1) were transferred to the indicated recipient mice (CD45.2) followed by NP-OVA/Alum immunization, and transferred OT-II cells were analyzed at day 7. (C) Recovered total OT-II cell number (top), frequency of Tfh cells among OT-II (middle), and total OT-II Tfh cell number (bottom) from the spleen are shown. (D) Representative flow cytometry profile of splenic OT-II cells (CD4+ B220− CD45.1+ Vα2+) analyzed for the Tfh CXCR5high PD-1high phenotype. Unimmunized, n = 4; MHCIIflox/flox, n = 5; and DC-MHCII cKO, n = 6. Data shown are the combined result of two independent experiments. (E) Cell-intrinsic requirement of MHCII on B cells for antigen-specific GC development. MHCII WT (CD45.1) + MHCII KO (CD45.2) mixed BM chimeric mice and control MHCII WT (CD45.1) + MHCII WT (CD45.2) BM chimeric mice were prepared and immunized with NP-KLH. At day 7 after immunization, chimerism of total B cells (B220+; left) and NP-binding GC B cells from the spleen (B220+ GL7+ Fas+; right) was analyzed. Each group, n = 4. Data are representative of two independent experiments. (F) Cell-intrinsic requirement of MHCII on B cells for antigen-specific IgG1 production. MHCII WT (Igha) and MHCII KO (Ighb) mixed BM chimera mice and control MHCII WT (Igha) and MHCII WT (Ighb) BM chimera mice were prepared and immunized with NP-KLH/Alum. At 3 wk after immunization, anti–NP-IgG1 titers were analyzed by ELISA. Each group, n = 3. Data are representative of two independent experiments. (G and H) BM chimeras were reconstituted by a mixture of B cell KO (μMT) + MHCII KO BM (MHCII−/−+ μMT; Fig. S2 E), resulting in the selective absence of MHCII on all B cells. Control chimeras were reconstituted by a mixture of B cell KO (μMT) + MHCII WT BM (MHCII+/+ + µMT) BM. OT-II T cells (CD45.1) were transferred to recipient BM chimera mice (CD45.2) followed by NP-OVA/Alum immunization 1 d later, and splenic OT-II cells were analyzed at day 8 after immunization. (G) Recovered total OT-II cell number (top), frequencies of OT-II Tfh cells (middle), and number of total OT-II Tfh cells were analyzed (bottom). ns, not significant. (H) Representative flow cytometry plot of OT-II cells (CD4+ B220− CD45.1+ Vα2+) analyzed for the Tfh CXCR5high PD-1high phenotype. Data presented are the combined result of three independent experiments. The total numbers of mice in the three combined experiments are unimmunized, n = 4; (MHCII+/+ + µMT) BM chimera, n = 3; and (MHCII−/− + μMT) BM chimera, n = 5. Statistical significance was evaluated by Student’s t test. All error bars represent mean ± SEM.