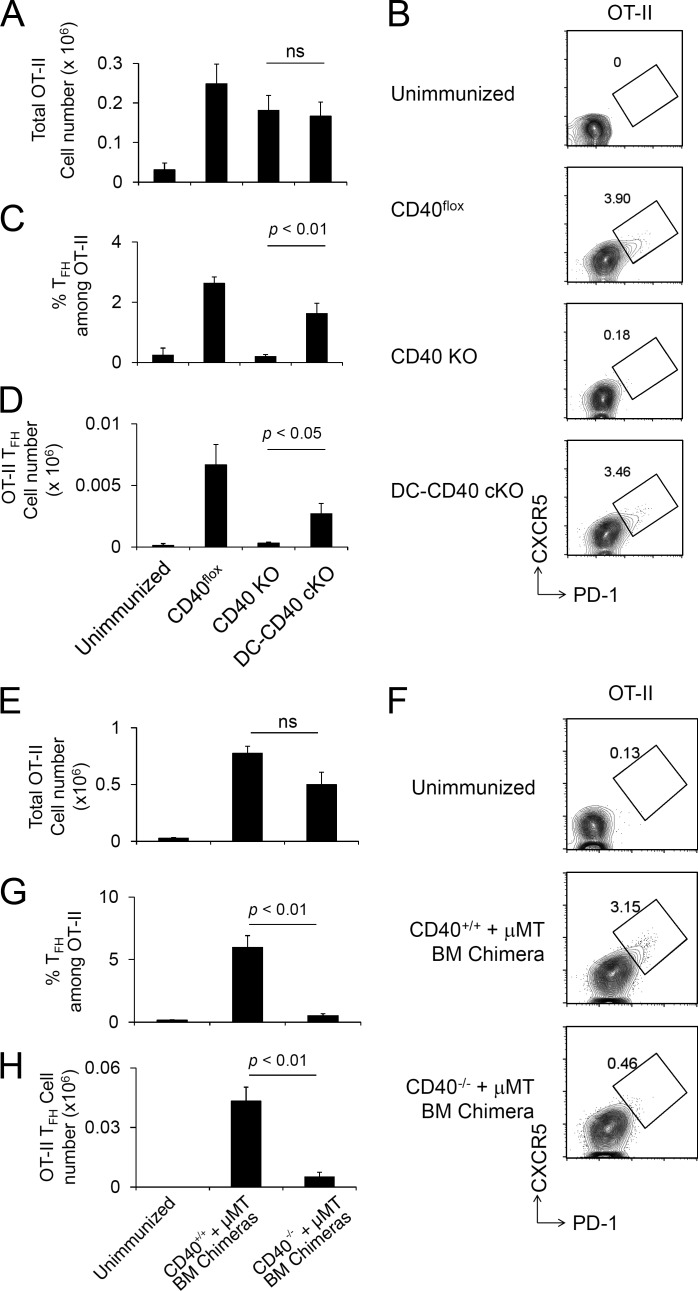
Figure 5.
Antigen-specific T cell activation and Tfh cell development require CD40 on B cells but not DCs. (A–D) Naive OT-II CD4+ T cells were transferred to CD40 cKO mice followed by NP-OVA/Alum immunization 1 d later, and splenic OT-II cells were analyzed at day 8 after immunization. Recovered total OT-II cell number (A), FACS plot of OT-II (CD4+ B220− CD45.1+ Vα2+) cells analyzed for Tfh CXCR5high PD-1high phenotype (B), frequency of Tfh cells among OT-II (C), and total OT-II Tfh cell number were determined (D). Data are combined from three independent experiments. The total numbers of mice in the three combined experiments are CD40flox, n = 8; CD40 KO, n = 8; and DC-CD40 cKO, n = 11. ns, not significant. (E–H) A mixture of BM from B cell KO and CD40 KO donors was used to reconstitute irradiated B cell KO host mice, generating chimeras in which all B cells were constitutively CD40 deficient (CD40−/− + µMT). Control chimeras received a mixture of BM from B cell KO and WT donors (CD40+/+ + µMT). OT-II T cells were transferred to recipient BM chimera mice (CD45.2) followed by NP-OVA/Alum immunization 1 d later, and splenic OT-II cells were analyzed at day 8 after immunization. Recovered OT-II cell number (E), FACS plot of OT-II (CD4+ CD45.1+ Vα2+) cells analyzed for the Tfh CXCR5highPD-1high phenotype (F), frequency of OT-II Tfh cells among total CD4+ T cells (G), and the number of Tfh phenotype OT-II cells were analyzed (H). Data presented are the combined result of three independent experiments. The total numbers of mice in the three combined experiments are unimmunized, n = 3; (CD40+/+ + µMT) BM chimera, n = 3; and (CD40−/− + μMT) BM chimera, n = 5. Statistical significance was determined by Student’s t test. All error bars represent means ± SEM.