Figure 3.
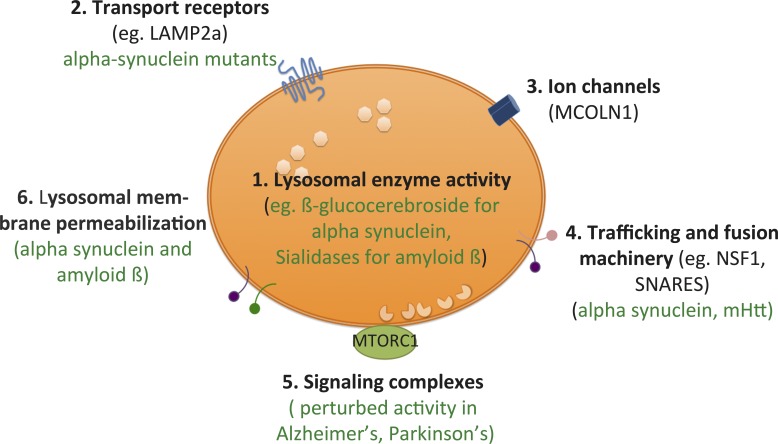
Multiple factors within lysosome structures can affect proteopathic disease propagation. Lysosomes are complex vesicular structures that regulate cellular homeostasis as well as perform a significant part of cellular degradation. (1) Lysosomal enzyme activity may decrease with the natural processes of aging or by the accumulation of resistant protein aggregates within the structure. The resultant diminished degradative capacity can lead to increased intracellular burden, not only of the toxic aggregate but also of partially degraded/oxidized lipids such as cholesterol, resulting in lysosomal stress and buildup of ROS. Insufficiency in β-glucocerebroside, for example, is implicated in α-synuclein accumulation. (2) The cytosolic tails of transport receptor proteins such as Lamp2a are involved in the recognition of protein substrates of chaperone-mediated autophagy. Recognition of the misfolded protein results in translocation of the protein across the lysosomal membrane to be degraded. However, misfolded α-synuclein mutant aggregates can bind to Lamp2a receptors and block their ability to transport, thereby causing decreased degradation and increased cytosolic burden of aggregates that can result in neurotoxicity through a variety of pathways. Other receptors have yet to be investigated. (3) Calcium channels such as MCOLN1 are involved in mediating lysosomal exocytosis. They have been identified as being affected in lysosomal storage diseases, dysregulating normal Ca2+ homeostasis in the cell. Although Ca2+ homeostasis is disturbed in these diseases, it remains unclear whether channels like these are directly perturbed by the buildup of protein. (4) Proteins involved in membrane fusion, such as NSF1, have been shown to be important for clearance of toxic protein aggregates such as Htt. These proteins may regulate fusion with autophagosomes or with the plasma membrane for lysosomal exocytosis. Aggregate buildup on the surface also affects trafficking of these vesicles. (5) Lysosome–nucleus signaling is regulated by nutrient-sensing machinery, which includes the mTORC1 complex. Binding of the master transcription factor TFEB to mTORC1 normally sequesters TFEB in the cytoplasm. However, upon certain signals such as nutrient starvation, TFEB is released, whereupon it enters the nucleus and up-regulates genes involved in lysosome biogenesis, lysosomal exocytosis and autophagy. Synuclein has been shown to bind directly to TFEB, and therefore, cytosolic aggregates can potentially dysregulate this signaling during neurodegenerative disease. (6) Direct breaching of the integrity of the lysosomal membrane by aggregates has been proposed to cause lysosomal damage, release of aggregates into the cytosol thereby leading to propagation of seeding as well as causing apoptosis through the release of cathepsins into the cytoplasm. Adapted from Settembre et al. (2013).