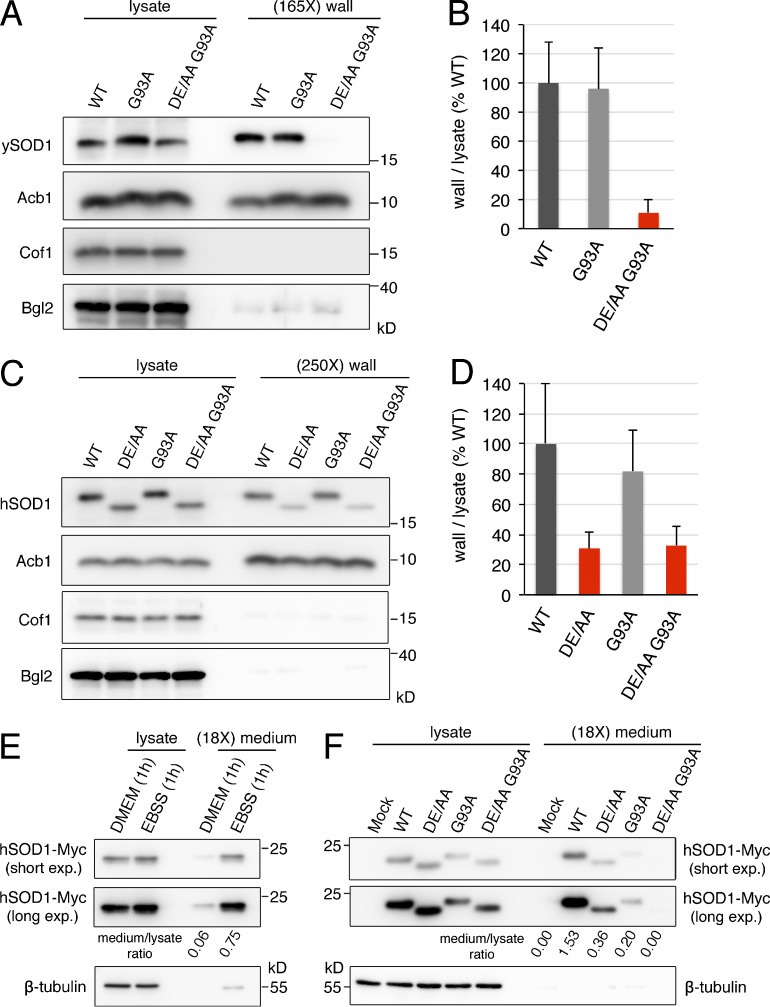
Figure 3.
The diacidic residues are necessary for secretion of wild-type and the ALS mutant of human SOD1. (A and B) The yeast equivalent to the ALS-linked mutant G93A of human SOD1 is secreted in response to starvation in a diacidic sequence-dependent manner. Strains expressing the indicated versions of yeast SOD1 were grown to mid-logarithmic phase, washed twice, and incubated in 2% potassium acetate for 2.5 h. Cell wall proteins were extracted from equal numbers of cells followed by TCA precipitation. Lysates and cell wall fractions were analyzed by Western blotting. The ratios of wall to lysate SOD1 were determined and represented as percentages of the strain expressing wild-type SOD1. (165X) wall indicates that the wall sample represents the material from 165-fold the number of cells analyzed in the lysate sample. Results are shown as means ± SEM of four experiments. (C and D) The DE77/78 sequence is required for secretion of wild-type and G93A human SOD1 by yeast cells. The ratio of wall to lysate of human SOD1 in yeast strains expressing the indicated versions of human SOD1 incubated in 2% potassium acetate for 2.5 h was determined as in A and B. (250X) wall indicates that the wall sample represents the material from 250-fold the number of cells analyzed in the lysate sample. Results are shown as means ± SEM of three experiments. (E) Secretion of wild-type human SOD1 is induced by EBSS starvation in HeLa cells. HeLa cells were transfected with a vector encoding wild-type human SOD1-Myc. After 24 h, cells were washed and incubated in either serum-free DMEM or EBSS (starvation medium) for 1 h. Conditioned media were collected and precipitated with TCA. Lysates and medium fractions were analyzed by Western blotting with anti-Myc and anti–β-tubulin antibodies. (F) The DE77/78 motif determines secretion of wild-type and G93A human SOD1 by HeLa cells. HeLa cells transfected with either an empty Myc vector (Mock) or plasmids encoding the indicated versions of Myc-tagged human SOD1 were incubated in EBSS for 1 h, and the levels of SOD1-Myc and β-tubulin in the lysates and medium fractions were analyzed as in E. (18X) medium indicates that the medium sample represents the material from 18-fold the number of cells analyzed in the lysate sample. Two different exposures (short and long) of the anti-Myc blots are shown. The ratio of medium to lysate of SOD1-Myc for each condition was determined and is indicated under the corresponding lane of the anti-Myc blots. The results of the secretion assays in HeLa cells are representative of two experiments.