SUMMARY
Chronic wasting disease (CWD) affects cervids and is the only known prion disease readily transmitted among free-ranging wild animal populations in nature. The increasing spread and prevalence of CWD among cervid populations threaten the survival of deer and elk herds in North America, and potentially beyond. This review focuses on prion ecology, specifically that of CWD, and the current understanding of the role that the environment may play in disease propagation. We recount the discovery of CWD, discuss the role of the environment in indirect CWD transmission, and consider potentially relevant environmental reservoirs and vectors. We conclude by discussing how understanding the environmental persistence of CWD lends insight into transmission dynamics and potential management and mitigation strategies.
KEYWORDS: prions, chronic wasting disease, cervids, soil, water, plants, ecology, environment, transmission
INTRODUCTION
Transmissible spongiform encephalopathies (TSEs) are a group of diseases caused by a unique infectious agent, the prion. The prion hypothesis asserts that prions arise from the misfolding of a normal host protein, the cellular prion protein (PrPC), into an abnormal, pathological isoform resistant to protease degradation (PrPRES) (1). Amyloid deposits of PrPRES and spongiform degeneration in the brain characterize TSEs (2). Clinical signs can vary among TSEs and include wasting, increased salivation, and general motor impairment. Prions, but not PrPC, resist inactivation by ionizing radiation, formalin, protease and nuclease treatment, and even autoclaving. Numerous TSEs exist that affect humans and other animals. Chronic wasting disease (CWD) is an animal TSE that affects cervids, such as elk (Cervus candensis), deer (Odocoileus hemionus), moose (Alces alces), caribou, and reindeer (Rangifer tarandus), and has become endemic in both free-ranging and captive herds (3–5). The exact mechanisms of CWD spread remain unclear, but experimental evidence and mathematical models support a role for environmental reservoirs and, potentially, vectors in CWD transmission dynamics (6, 7) (Fig. 1). Population densities and contact frequencies can also influence CWD spread and transmission (8–10). Water, soil, feces, fomites, and plants may act as environmental reservoirs (7, 11–14). Continued spread in free-ranging populations, the recent discovery of CWD in Norway (15), and purported long-term outcomes forecast possible extinction events. The survival of cervid populations worldwide depends on understanding the role of the environment in CWD transmission for the development of effective surveillance, containment, and mitigation strategies.
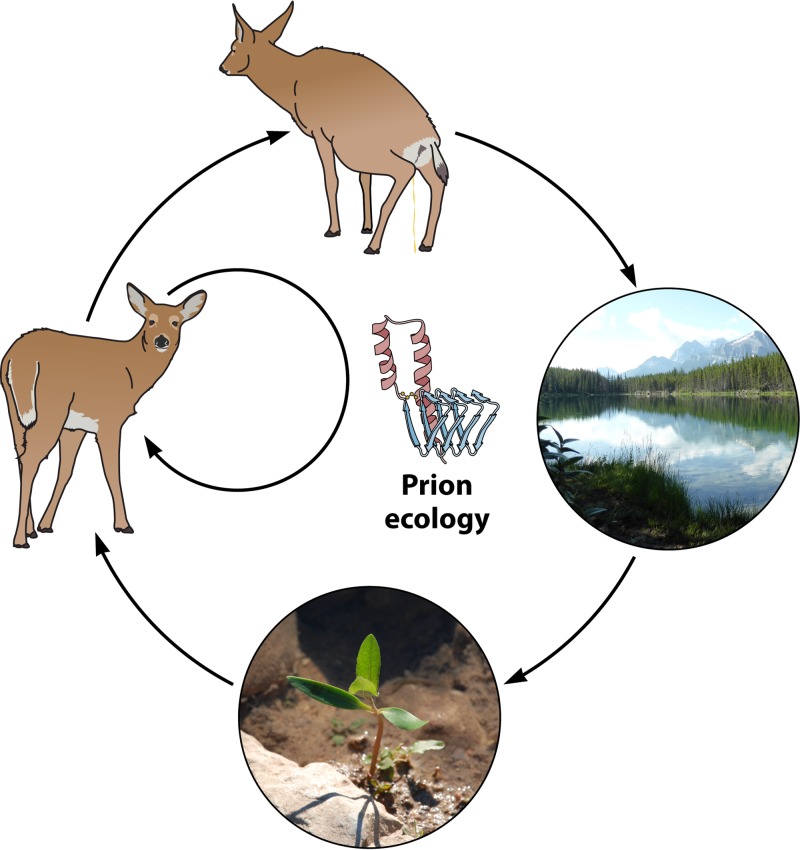
FIG 1.
CWD prion ecology. Shown is the potential movement of CWD prions in the environment. While direct transmission likely contributes the most to CWD spread, indirect transmission also occurs via CWD prion deposition into the environment from urine, feces, and saliva onto and into water, soil, and plants. Cervids and other animals likely consume prions contained in these reservoirs and become infected.
THE EMERGENCE OF CWD
The discovery of CWD happened incrementally, and over this time, CWD became endemic in both free-ranging and captive animal herds. A population of captive deer being held in wildlife facilities in Colorado from 1967 to 1979 were noted to have been experiencing the same group of clinical signs, including weight loss, torpor, polydipsia, polyuria, low urine specific gravity, and hypotonia. Histopathological changes were noted in brain tissue and included spongiform degeneration and vacuolization (3). There were similarities to other TSEs, both human and animal, but no evidence of CWD transmission, only some indirect contact between animals at fence lines. Two years later, the same researchers reported disease phenotypes in Rocky Mountain elk and in free-ranging cervids as well (16). Amyloid plaques were also noted in the brains of affected deer (17). Intracranial inoculation of brain homogenates from affected deer into naive deer and ferrets transmitted CWD after an incubation time of 17 to 24 months (4, 18). Prion amyloids found in the brains of CWD-affected elk and deer provided evidence of CWD sharing the same etiology and pathology as those of other TSEs (19, 20).
The origin of CWD is still widely contested. It remains uncertain whether CWD arose spontaneously as a sporadic incident in one or a few free-ranging or captive deer and then spread to other cervids across landscapes. No evidence demonstrates CWD being caused by infected feed or feed products, as occurred in cattle to cause bovine spongiform encephalopathy (BSE) (21, 22). The scrapie-BSE nexus drives one popular hypothesis that scrapie crossed the species barrier from sheep to deer when animals were housed concurrently in Colorado facilities. It may then have spread to and from free-ranging animals when they came into fence-line contact with captive animals (4). Most, if not all, CWD cases can be tracked back to movement of infected captive cervids from the initial epicenter in northern Colorado and southern Wyoming to game farms throughout North America and South Korea, supporting that hypothesis. While not linked to CWD emergence in North America, new CWD cases recently discovered in Norway (15) may be attributed to free-ranging reindeer and moose acquiring the disease from sympatric scrapie-infected sheep. However, the relatively facile spontaneous conversion of cervid PrPC to CWD prions in vitro (23) suggests that CWD may occur spontaneously, like scrapie or sporadic Creutzfeldt-Jakob disease (CJD).
Numerous PRNP gene polymorphisms increase susceptibility or resistance to disease in deer and elk. The most common mule deer genotypes at codons 95, 96, 132, 138, and 225 encode glutamine (Q), glycine (G), serine (S), methionine (M), and serine (S), respectively. Less common polymorphisms include histidine (H) at codon 95, S96, leucine (L) at codon 132, asparagine (N) at codon 138, and phenylalanine (F) at codon 225. Transgenic mouse experiments convincingly show that the more common genotypes correlate with increased susceptibility to CWD (24–26). Comparisons between free-ranging and captive Rocky Mountain elk linked CWD susceptibility to M132 homozygosity (25). Most genotyped CWD-negative deer from Wisconsin expressed genotypes linked to CWD-positive deer, revealing a huge deer population susceptible to CWD (24). Evidence that resistant genotypes provide delayed onset or prolonged incubation periods has been shown, but these genotypes are rarely found in large populations (27–29). These data alert surveillance and other wildlife management agencies to be aware of the susceptible populations in their areas and to possibly mitigate continued spread based on this information.
ROLE FOR THE ENVIRONMENT IN INDIRECT CWD TRANSMISSION
In the 1940s, shepherds in northern Iceland culled their entire sheep population in an effort to eradicate Maedi, an ovine lung disease rampant in their flocks. The area in which the culling took place overlapped areas where evidence supported the presence of scrapie, the prion disease of sheep. Healthy sheep from an area with no evidence of scrapie were introduced into this area. After 3 years, the new animals became sick with scrapie (30–32). In 1978, Iceland again tried to eradicate scrapie by imposing strict implementations, including culling positive and at-risk animals, disinfecting or destroying buildings that housed infected animals, banning translocation of animals and equipment from positive farms, restocking farms after at least two dormant years, and continued surveillance. After 16 years, some of the reintroduced flocks developed clinical scrapie (30). Because these flocks had been scrapie-free through several generations, the environmental persistence of scrapie prions on these farms likely contributed to the emergence of new scrapie cases in these sheep.
Similar environmental persistence has been noted with CWD. The Foothills Wildlife Research Facility (FWRF) in Fort Collins, CO, where CWD was originally detected, underwent a similar cleansing and culling in order to eradicate CWD and to establish new, CWD-free herds. Approximately 2 years after reintroduction of elk herds to the FWRF, animals began showing clinical signs of illness. These decontamination failures implicated environmental reservoirs in the reemergence of CWD in these herds, all of whose members were acquired from Rocky Mountain National Park as calves, born to wild free-ranging dams, when they were less than 1 week old (33). While the reoccurrence of infection may have been from environmental persistence and indirect transmission of cervid prions, vertical transmission from positive mothers infecting their calves also may have occurred. As CWD progressed, animals may have begun shedding prions, leading to both a direct contact source between elk and an indirect source via accumulation in paddocks. An experimental demonstration of indirect environmental reservoir transmission was shown in 2004, when uninfected mule deer were housed in paddocks containing either infected deer or a positive deer carcass decomposed 20 months prior or in an empty paddock containing excreta from positive deer housed 26 months prior. Uninfected deer became CWD positive in all three scenarios (34). CWD transmission increases during winter, possibly due to density-dependent transmission. Deer herds stay together over winter, which may increase the chance of both direct transmission and contacting accumulated positive environmental material. Indeed, the prevalence of CWD has been shown to be much higher in defined winter ranges, which may be due to an accumulation of PrP-positive material in these areas (35).
Bedding and water transferred from indoor rooms housing CWD-positive deer transmitted CWD to negative deer in prion-naive rooms in only 15 months. Tonsil biopsy confirmed the presence of PrPCWD in one of two animals (13). Both animals eventually contracted CWD solely through environmental exposure. Given the overwhelming evidence that prions resist degradation, persist in the environment for years (30), and can be shed in excreta of clinical as well as asymptomatic animals (12, 36–39), CWD prions likely accumulate in the environment. Estimation of prion titers by mouse bioassay of CWD prions intracerebrally inoculated into susceptible animals indicates that a single experimentally inoculated deer can defecate 10.9 log 50% lethal dose (LD50) units of CWD prions over a 10-month period, a typical duration of CWD (39). The same deer can also urinate up to 6 log LD50 units of prions into the environment over this time. Naturally infected deer defecate an estimated 1,000 LD50 units of CWD prions daily (12). While these doses are relatively low, repeated frequent exposure, either direct or indirect, increases the likelihood of contracting CWD (7, 40).
POTENTIAL ENVIRONMENTAL PRION RESERVOIRS AND VECTORS
Researchers are currently investigating at least three potential environmental reservoirs for prions—soil, water, and plants. These proposed reservoirs most likely accumulate prions deposited from excreta and decaying carcasses. Other fomites in the environment, such as salt licks, wallows, fences, bedding sites, and even buildings, may also contribute to prion deposition in the environment (13, 30, 41, 42).
Deer and elk ingest small but appreciable amounts of soil (<2% soil consumption in the diet) (7, 43). They often ingest soil inadvertently while feeding, but they may also intentionally eat soil to obtain micronutrients essential to their metabolism. These considerations led researchers to begin looking for prions in soil and to determine whether PrPC binds soil and/or its constituents and if the prion infectivity and/or conformation changes in a soil environment. One study looked at the potential ability of PrPC to misfold into the pathological prion structure when bound to a mineral-phase soil component known as montmorillonite (MTE) (44). While PrP-MTE complexes were formed and some α-helical-to-β-sheet-like structural changes occurred, these structural transformations were distinct from the pH-induced conformational changes that occur during prion formation and did not produce infectious prions. Similar work demonstrated that prions could also adsorb to MTE, microparticles of quartz, kaolinite (another mineral-phase soil component), and a variety of whole soils (45). MTE bound prions so tightly that 10% sodium dodecyl sulfate was required for desorption and resulted in N-terminal truncation of PrPRES. Prions present in a complex matrix of infected brain homogenate adsorbed to MTE much more slowly, which likely mimics environmental contamination (46). Experimental evidence shows that unbound prions degrade over time, while soil-bound prions remain at stable or increasing levels (47, 48), suggesting that prions remain stable in the environment when bound to soil or clay components and potentially become more infectious. Prions remained infectious when bound to MTE and inoculated intracranially into rodents (49). MTE was recently shown to increase the environmental stability and bioavailability of prions bound to it (7). However, when prions were subjected to simulated weather conditions, such as heating, wetting, and drying, MTE actually potentiated prion degradation (50). Microbial communities in soil, compost, and lichens also demonstrate significant reductions of prion titers (51–53). Thus, natural and cultivated microbial communities may mitigate some, but not all, environmental prion contamination.
Prions that do survive environmental insult maintain or even augment prion transmission. Prion infectivity and oral transmission increased when PrP was bound to soil (54). Intranasal inoculations of deer with prions bound to MTE dust particles resulted in efficient transmission of CWD (55). Soil-bound prions resist rumen digestion, and MTE enhances the bioavailability and retention of prions bound to it (56). This high affinity of MTE for prions potentially may be exploited as a therapeutic or decontaminant to remove PrP from complex solutions.
Nichols et al. found PrPRES in water collected from an area in Colorado where CWD is endemic and from raw water samples collected in a nearby water treatment facility (11). BSE prions survived in raw sewage, with little or no reduction in infectivity (57), and organic matter present in water partially prevented degradation of PrPRES and loss of infectivity (58, 59). Fomites from infected deer, including water, transmitted CWD prions to uninfected deer with no direct contact with infected cohorts (13).
Detection of prions in soil, water, excreta, and decaying carcasses on the landscape raises legitimate concern about whether plants, the main food source of deer and elk, can act as prion vectors by active uptake or passive contamination. Plants take up protein, as a nitrogen source, and other nutrients in their roots, stems, and aerial tissues (60–62). Endophytic bacteria and bacterial communities that fix nitrogen and fight plant diseases have been described (61, 63, 64). Plants may conceivably take up prions from soil or water into their root systems or aerial tissues or become surface contaminated through saliva, urine, feces, and/or decaying CWD-infected carcasses. An attempt was made to assess the potential of wheat grass grown in agar medium to take up prions from water (65). Both roots and lower stems were examined for PrPRES by using commercially available enzyme-linked immunosorbent assays for PrPRES and an ultrasensitive prion detection assay, i.e., protein misfolding cyclic amplification (PMCA). The researchers reported finding PrPRES inside roots but not stems, in addition to PrPRES on stem and root surfaces that was rinsed away with water. PMCA experiments were inconclusive due to nonspecific amplification in control unexposed plant homogenates. Prion uptake was assessed for only one 24-h time point, and significant contamination issues were reported. Another group successfully used PMCA to detect prions taken into roots, stems, and leaves by wheat grass plants (Triticum aestivum L.) grown with high concentrations of prions spiked into soil (14). Since copious data show that soil binds prions extremely tightly, the mechanism by which prions move from soil to plants remains unclear. Perhaps the experimental conditions used soil saturated with prions and facilitated plant uptake of free prions. If so, these results may not be ecologically relevant, since one expects very low levels of prion contamination, except perhaps in areas just under a decaying infected carcass. More relevant experimental contamination of plants by spraying of infected brain homogenate onto wheat (Triticum aestivum L.) leaves resulted in detection of PrPRES at a stable level for 49 days. Different plant tissues were also exposed to urine and feces from both CWD-positive animals and scrapie-infected hamsters, and the results again showed prions bound to the plant tissues after rinsing and drying.
Decaying carcasses of any kind affect the ecosystem around them, often leading to higher concentrations of nitrogen and a difference in plant species in the area that may be present for years after the initial decomposition. As a carcass decays, the body fluids released destroy the plants underneath and in the surrounding area, creating a zone of disturbance which, after time, becomes zones of fertility due to nutrients and limited competition from other species (66). Since CWD prions have been shown to persist in the environment, it is postulated that a decaying CWD-positive carcass can saturate the environment with prions, which can then be taken up into plants as growth of new flora occurs. Prions were detected in roots, stems, and leaves of wheat plants (Hordeum vulgar) grown in soil experimentally contaminated with prions (14). Cervids readily eat both wheat and barley grasses in the spring: around 4 to 64% of the mule deer diet is composed of grasses, while the remainder comes from shrubs and trees (67).
Reservoir or vector animals transmit many emerging infectious diseases that affect wildlife populations. No reservoir animals have been found for CWD, although several vector animals, including predators and scavengers, may aid in dissemination of CWD prions across the landscape. Coyotes (68), cougars (69), and even crows (70) have been investigated as potential CWD reservoirs and/or vectors. Experimentally inoculated coyotes and crows both shed infectious prions in their feces. Both mammalian and avian scavengers travel scores of kilometers per day and likely contribute to prion dissemination across their habitats and prion accumulation across landscapes. While cougars prey more successfully on CWD-affected cervids than on unaffected cervids, no evidence exists that cougars have contracted feline spongiform encephalopathy as a result. Thus, if they contribute to prion dissemination, they likely do so as vectors, not reservoirs.
Even if reservoir species do exist, they likely replicate prions as a new strain that likely exhibits altered host ranges and introduces new species barriers to cervids and other mammals that contact them. But Bian et al. recently demonstrated nonadapted prion amplification (NAPA) experimentally in vitro and in vivo (71), so host range restriction by reservoir animals may not be absolute. If NAPA occurs in nature, prion dissemination may aid environmental prion reservoirs to perpetuate CWD via indirect transmission. In the absence of CWD reservoir animals, translocation of CWD-infected cervids often facilitates emergence, because it can bring susceptible naive animals in contact with infected ones and their contaminated environments (72), providing proximity to CWD prion reservoirs for both direct and indirect transmission.
MODELING INDIRECT PRION TRANSMISSION DYNAMICS
Mathematical models of CWD prevalence and dynamics support the hypothesis that direct transmission certainly accounts for most transmission events, with both population density and contact frequency contributing to CWD spread (9, 73). However, modeling of only direct transmission failed to account for data showing high and increasing prevalences in areas of endemicity (74, 75). Modeling of exposure risks of sheep flocks to scrapie identified buildings and pastures as likely sources of indirect scrapie transmission (76). Models of CWD transmission that include both direct and indirect parameters more closely match the available data on current epidemics seen in Colorado, Wyoming, and Wisconsin (6, 10). Miller et al. compared seven different CWD transmission models and found that those including indirect transmission parameters matched existing data four times more accurately than those modeling only direct transmission (75). Including landscape effects derived from GPS data in areas of Alberta, Canada, where CWD is endemic increased the precision of models using density parameters (8). Bayesian models assuming a long prion half-life implicate a significant role for indirect CWD prion exposure in CWD transmission dynamics due to accumulating prions in the environment (74, 77). They also model a slowly progressing disease that predicts a potential nonfatal carrier state that may ameliorate population decline initially but accelerate transmission as environmental contamination mounts, a conclusion corroborated by another model considering prolonged environmental prion persistence (78). Repeated sampling of the same mule deer populations in Colorado informed another Bayesian hierarchical model that predicted a steady population decline that could be slowed by hunter harvest, vehicular deaths, and predation (6). Few data or models predict sex-dependent transmission, although one model predicted that sex-specific differences fit frequency-dependent transmission models better (9). However, the authors included no indirect transmission parameter in their model, which may not accurately fit existing data dependent on the level of environmental accumulation. CWD prevalence continues to increase from Wyoming to Wisconsin to Arkansas, with these areas of endemicity reporting estimates ranging from 15 to 50% for free-ranging populations (47). With CWD spreading unabated, prion loads accumulating and persisting on landscapes, and the prevalence continuing to increase, both mathematical models and epidemiological data suggest that a tipping point may be reached, potentially resulting in herd decimation and population decline, especially in the presence of environmental reservoirs that retain prions for extended periods.
MITIGATING ENVIRONMENTAL PRION CONTAMINATION
Continued spread of CWD is clearly a multifaceted event. Prion persistence, indirect transmission, genetics, population density, contact frequency, management strategies, and other unrealized factors all may affect CWD ecology. Emerging data support a possibly significant role of soil, water, feces, and plants as prion reservoirs contributing to environmental contamination and indirect CWD transmission. CWD has now been found in cervids in 22 U.S. states, 2 Canadian provinces, South Korea, and Norway (5, 15). As CWD continues to unabatedly establish endemicity wherever it appears, eliminating or reducing environmental prion loads across landscapes represents a critical but enormous challenge.
Researchers have demonstrated that composting, incineration, and enzyme treatments may help to degrade environmental PrPRES (53, 79, 80). These studies have focused mainly on specified risk material (SRM) generated from abattoirs and commercial meat processing plants. Brown et al. detected residual prion infectivity even after incineration at 600°C, although the initial prion titer was over 109 LD50 units/g of tissue (80), which is well beyond realistic environmental prion titers. Composting reduces prion titers in SRM by a much more modest 1 to 2 log, with cultivation of a proteolytic microbiome eliminating another order of magnitude of infectivity (53). Robust prion oxidation by ozone treatment also reduces prion infectivity in SRM and contaminated wastewater, by several orders of magnitude (81, 82). These procedures may be effective for reducing the likelihood of contaminating environments proximal to industrial farming enterprises but are impractical or simply cannot be applied to massive areas contaminated by CWD prions deposited by infected cervids across three continents. Although sources of CWD prions that contaminate endemic environments contain low levels of prions, continuous prion deposition and sustained prion persistence in environmental reservoirs pose significant challenges for bioremediation. Infected free-ranging animals continuously shedding prions in the environment and large host ranges complicate environmental decontamination strategies, especially if free-ranging infected animals cannot be removed, cordoned, or quarantined and contaminated landscapes protected from infected cervids returning to those habitats. Recontamination will likely occur and population decline will likely result if mitigation strategies fail. More sampling and surveillance need to be undertaken to understand the extent of environmental contamination in different areas of endemicity, and new mitigation strategies should be explored.
Controlled burning of landscapes in North America helps to mitigate fire danger in drought-stricken areas, in some of which CWD is endemic. Burning of plants, feces, and topsoil in these areas may reduce the low-level prion infectivity present in these areas. While the burn temperature and duration are certainly much lower than those attained in the experiments for which Brown et al. reported residual prion infectivity, naturally CWD-contaminated areas certainly contain many orders of magnitude less prion infectivity than prion-infected SRM. Prescribed burning may sufficiently lower prion titers on landscapes to at least impede the indirect transmission of CWD. Combined with directed hunter harvests, systematic culling, and targeted implementation of CWD vaccines (83, 84), we may be able to stem the slow but steady spread of CWD across the landscape.
ACKNOWLEDGMENTS
We thank Jan Leach, Jeff Wilusz, and Candace Mathiason for critical readings of this review.
Biographies

Mark Zabel earned his Ph.D. in experimental pathology in the lab of John H. Weis at the University of Utah School of Medicine, with training in prion biology, biochemistry, and immunology from Adriano Aguzzi at the Institüt for Neuropathology, Universitätspital Zürich, Zurich, Switzerland. He is currently Associate Director of the Prion Research Center and Professor in the Department of Microbiology, Immunology and Pathology, College of Veterinary Medicine and Biomedical Sciences, Colorado State University. Dr. Zabel collaborates with researchers at the USDA National Wildlife Research Center, the National Park Service, Colorado Parks and Wildlife, and Rocky Mountain National Park. These collaborators seek to understand CWD prevalence in cervids and prion movement in the environment, with the ultimate goal of developing vaccines, therapeutics, and CWD management and mitigation strategies to stem the tide of CWD spread across landscapes. Dr. Zabel enjoys the opportunities to work outside to achieve these goals.

Aimee Ortega earned her M.S. in microbiology in the Zabel laboratory at the Prion Research Center at Colorado State University, Department of Microbiology, Immunology and Pathology, College of Veterinary Medicine and Biomedical Sciences. She drove the project to evaluate the role of plants as a CWD prion reservoir in the environment and as vectors for indirect CWD transmission. She is currently a clinical research scientist at the Veterans Administration Medical Center in Denver, CO. Aimee's thesis committee encouraged her to write this review article as an extension of the introduction to her thesis. Aimee is an accomplished researcher, avid backpacker, and science advocate.
REFERENCES
- 1.Prusiner SB. 1982. Novel proteinaceous infectious particles cause scrapie. Science 216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 2.Beck E, Daniel PM. 1987. Neuropathology of transmissible spongiform encephalopathies, p 331–385. In Prusiner SB, McKinley MP (ed), Prions: novel infectious pathogens causing scrapie and Creutzfeldt-Jakob disease. Academic Press, San Diego, CA. [Google Scholar]
- 3.Williams ES, Young S. 1980. Chronic wasting disease of captive mule deer: a spongiform encephalopathy. J Wildl Dis 16:89–98. doi: 10.7589/0090-3558-16.1.89. [DOI] [PubMed] [Google Scholar]
- 4.Williams ES, Young S. 1992. Spongiform encephalopathies in Cervidae. Rev Sci Tech 11:551–567. doi: 10.20506/rst.11.2.611. [DOI] [PubMed] [Google Scholar]
- 5.Haley NJ, Hoover EA. 2015. Chronic wasting disease of cervids: current knowledge and future perspectives. Annu Rev Anim Biosci 3:305–325. doi: 10.1146/annurev-animal-022114-111001. [DOI] [PubMed] [Google Scholar]
- 6.Geremia C, Miller MW, Hoeting JA, Antolin MF, Hobbs NT. 2015. Bayesian modeling of prion disease dynamics in mule deer using population monitoring and capture-recapture data. PLoS One 10:e0140687. doi: 10.1371/journal.pone.0140687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wyckoff AC, Kane S, Lockwood K. 2016. Clay components in soil dictate environmental stability and bioavailability of cervid prions in mice. Front Microbiol 7:1885. doi: 10.3389/fmicb.2016.01885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Habib TJ, Merrill EH, Pybus MJ, Coltman DW. 2011. Modelling landscape effects on density-contact rate relationships of deer in eastern Alberta: implications for chronic wasting disease. Ecol Model 222:2722–2732. doi: 10.1016/j.ecolmodel.2011.05.007. [DOI] [Google Scholar]
- 9.Jennelle CS, Henaux V, Wasserberg G, Thiagarajan B, Rolley RE, Samuel MD. 2014. Transmission of chronic wasting disease in Wisconsin white-tailed deer: implications for disease spread and management. PLoS One 9:e91043. doi: 10.1371/journal.pone.0091043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Storm DJ, Samuel MD, Rolley RE, Shelton P, Keuler NS, Richards BJ, Van Deelen TR. 2013. Deer density and disease prevalence influence transmission of chronic wasting disease in white-tailed deer. Ecosphere 4:art10. doi: 10.1890/ES12-00141.1. [DOI] [Google Scholar]
- 11.Nichols TA, Pulford B, Wyckoff AC, Meyerett C, Michel B, Gertig K, Hoover EA, Jewell JE, Telling GC, Zabel MD. 2009. Detection of protease-resistant cervid prion protein in water from a CWD-endemic area. Prion 3:171–183. doi: 10.4161/pri.3.3.9819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pulford B, Spraker TR, Wyckoff AC, Meyerett C, Bender H, Ferguson A, Wyatt B, Lockwood K, Powers J, Telling GC, Wild MA, Zabel MD. 2012. Detection of PrPCWD in feces from naturally exposed Rocky Mountain elk (Cervus elaphus nelsoni) using protein misfolding cyclic amplification. J Wildl Dis 48:425–434. doi: 10.7589/0090-3558-48.2.425. [DOI] [PubMed] [Google Scholar]
- 13.Mathiason CK, Hays SA, Powers J, Hayes-Klug J, Langenberg J, Dahmes SJ, Osborn DA, Miller KV, Warren RJ, Mason GL, Hoover EA. 2009. Infectious prions in pre-clinical deer and transmission of chronic wasting disease solely by environmental exposure. PLoS One 4:e5916. doi: 10.1371/journal.pone.0005916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pritzkow S, Morales R, Moda F, Khan U, Telling GC, Hoover E, Soto C. 2015. Grass plants bind, retain, uptake, and transport infectious prions. Cell Rep 11:1168–1175. doi: 10.1016/j.celrep.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benestad SL, Mitchell G, Simmons M, Ytrehus B, Vikøren T. 2016. First case of chronic wasting disease in Europe in a Norwegian free-ranging reindeer. Vet Res 47:88. doi: 10.1186/s13567-016-0375-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams ES, Young S. 1982. Spongiform encephalopathy of Rocky Mountain elk. J Wildl Dis 18:465–471. doi: 10.7589/0090-3558-18.4.465. [DOI] [PubMed] [Google Scholar]
- 17.Bahmanyar S, Williams ES, Johnson FB, Young S, Gajdusek DC. 1985. Amyloid plaques in spongiform encephalopathy of mule deer. J Comp Pathol 95:1–5. doi: 10.1016/0021-9975(85)90071-4. [DOI] [PubMed] [Google Scholar]
- 18.Spraker TR, Miller MW, Williams ES, Getzy DM, Adrian WJ, Schoonveld GG, Spowart RA, O'Rourke KI, Miller JM, Merz PA. 1997. Spongiform encephalopathy in free-ranging mule deer (Odocoileus hemionus), white-tailed deer (Odocoileus virginianus) and Rocky Mountain elk (Cervus elaphus nelsoni) in northcentral Colorado. J Wildl Dis 33:1–6. doi: 10.7589/0090-3558-33.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Guiroy DC, Williams ES, Yanagihara R, Gajdusek DC. 1991. Immunolocalization of scrapie amyloid (PrP27-30) in chronic wasting disease of Rocky Mountain elk and hybrids of captive mule deer and white-tailed deer. Neurosci Lett 126:195–198. doi: 10.1016/0304-3940(91)90552-5. [DOI] [PubMed] [Google Scholar]
- 20.Guiroy DC, Marsh RF, Yanagihara R, Gajdusek DC. 1993. Immunolocalization of scrapie amyloid in non-congophilic, non-birefringent deposits in golden Syrian hamsters with experimental transmissible mink encephalopathy. Neurosci Lett 155:112–115. doi: 10.1016/0304-3940(93)90685-E. [DOI] [PubMed] [Google Scholar]
- 21.Parodi AL, Brugere Picoux J, Chatelain J, Laplanche JL. 1990. Bovine spongiform encephalopathy: a new entity caused by a non-conventional transmissible agent. Bull Acad Natl Med 174:731–739. [PubMed] [Google Scholar]
- 22.Pattison IH. 1991. Origins of BSE. Vet Rec 128:262–263. [DOI] [PubMed] [Google Scholar]
- 23.Meyerett-Reid C, Wyckoff AC, Spraker T, Pulford B, Bender H, Zabel MD, Imperiale MJ. 2017. De novo generation of a unique cervid prion strain using protein misfolding cyclic amplification. mSphere 2:e00372-16. doi: 10.1128/mSphere.00372-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson C, Johnson J, Clayton M, McKenzie D, Aiken J. 2003. Prion protein gene heterogeneity in free-ranging white-tailed deer within the chronic wasting disease affected region of Wisconsin. J Wildl Dis 39:576–581. doi: 10.7589/0090-3558-39.3.576. [DOI] [PubMed] [Google Scholar]
- 25.O'Rourke KI, Besser TE, Miller MW, Cline TF, Spraker TR, Jenny AL, Wild MA, Zebarth GL, Williams ES. 1999. PrP genotypes of captive and free-ranging Rocky Mountain elk (Cervus elaphus nelsoni) with chronic wasting disease. J Gen Virol 80:2765–2769. doi: 10.1099/0022-1317-80-10-2765. [DOI] [PubMed] [Google Scholar]
- 26.Raymond GJ, Bossers A, Raymond LD, O'Rourke KI, McHolland LE, Bryant PK, Miller MW, Williams ES, Smits M, Caughey B. 2000. Evidence of a molecular barrier limiting susceptibility of humans, cattle and sheep to chronic wasting disease. EMBO J 19:4425–4430. doi: 10.1093/emboj/19.17.4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jewell JE, Conner MM, Wolfe LL, Miller MW, Williams ES. 2005. Low frequency of PrP genotype 225SF among free-ranging mule deer (Odocoileus hemionus) with chronic wasting disease. J Gen Virol 86:2127–2134. doi: 10.1099/vir.0.81077-0. [DOI] [PubMed] [Google Scholar]
- 28.Johnson C, Johnson J, Vanderloo JP, Keane D, Aiken JM, McKenzie D. 2006. Prion protein polymorphisms in white-tailed deer influence susceptibility to chronic wasting disease. J Gen Virol 87:2109–2114. doi: 10.1099/vir.0.81615-0. [DOI] [PubMed] [Google Scholar]
- 29.Kelly AC, Mateus-Pinilla NE, Diffendorfer J, Jewell E, Ruiz MO, Killefer J, Shelton P, Beissel T, Novakofski J. 2008. Prion sequence polymorphisms and chronic wasting disease resistance in Illinois white-tailed deer (Odocoileus virginianus). Prion 2:28–36. doi: 10.4161/pri.2.1.6321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Georgsson G, Sigurdarson S, Brown P. 2006. Infectious agent of sheep scrapie may persist in the environment for at least 16 years. J Gen Virol 87:3737–3740. doi: 10.1099/vir.0.82011-0. [DOI] [PubMed] [Google Scholar]
- 31.Palsson PA. 1980. Rida (scrapie) in Icelandic sheep and its epidemiology. Acta Neurol Scand 62:25–32. [Google Scholar]
- 32.Sigurðsson B. 1954. Rida, a chronic encephalitis of sheep: with general remarks on infections which develop slowly and some of their special characteristics. Brit Vet J 1954:341–354. [Google Scholar]
- 33.Miller MW, Wild MA, Williams ES. 1998. Epidemiology of chronic wasting disease in captive Rocky Mountain elk. J Wildl Dis 34:532–538. doi: 10.7589/0090-3558-34.3.532. [DOI] [PubMed] [Google Scholar]
- 34.Miller MW, Williams ES, Hobbs NT, Wolfe LL. 2004. Environmental sources of prion transmission in mule deer. Emerg Infect Dis 10:1003–1006. doi: 10.3201/eid1006.040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller MW, Conner MM. 2005. Epidemiology of chronic wasting disease in free-ranging mule deer: spatial, temporal, and demographic influences on observed prevalence patterns. J Wildl Dis 41:275–290. doi: 10.7589/0090-3558-41.2.275. [DOI] [PubMed] [Google Scholar]
- 36.Haley NJ, Mathiason CK, Zabel MD, Telling GC, Hoover EA. 2009. Detection of sub-clinical CWD infection in conventional test-negative deer long after oral exposure to urine and feces from CWD+ deer. PLoS One 4:e4848. doi: 10.1371/journal.pone.0004848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henderson DM, Denkers ND, Hoover CE, Garbino N, Mathiason CK, Hoover EA. 2015. Longitudinal detection of prion shedding in saliva and urine by chronic wasting disease-infected deer by real-time quaking-induced conversion. J Virol 89:9338–9347. doi: 10.1128/JVI.01118-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seeger H, Heikenwalder M, Zeller N, Kranich J, Schwarz P, Gaspert A, Seifert B, Miele G, Aguzzi A. 2005. Coincident scrapie infection and nephritis lead to urinary prion excretion. Science 310:324–326. doi: 10.1126/science.1118829. [DOI] [PubMed] [Google Scholar]
- 39.Tamgüney G, Miller MW, Wolfe LL, Sirochman TM, Glidden DV, Palmer C, Lemus A, DeArmond SJ, Prusiner SB. 2009. Asymptomatic deer excrete infectious prions in faeces. Nature 461:529–532. doi: 10.1038/nature08289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diringer H, Roehmel J, Beekes M. 1998. Effect of repeated oral infection of hamsters with scrapie. J Gen Virol 79:609–612. doi: 10.1099/0022-1317-79-3-609. [DOI] [PubMed] [Google Scholar]
- 41.Vercauteren KC, Burke PW, Phillips GE, Fischer JW, Seward NW, Wunder BA, Lavelle MJ. 2007. Elk use of wallows and potential chronic wasting disease transmission. J Wildl Dis 43:784–788. doi: 10.7589/0090-3558-43.4.784. [DOI] [PubMed] [Google Scholar]
- 42.Maddison BC, Baker CA, Terry LA, Bellworthy SJ, Thorne L, Rees HC, Gough KC. 2010. Environmental sources of scrapie prions. J Virol 84:11560–11562. doi: 10.1128/JVI.01133-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beyer WN, Connor EE, Gerould S. 1994. Estimates of soil ingestion by wildlife. J Wildl Manage 58:375. doi: 10.2307/3809405. [DOI] [Google Scholar]
- 44.Revault M, Quiquampoix H, Baron MH, Noinville S. 2005. Fate of prions in soil: trapped conformation of full-length ovine prion protein induced by adsorption on clays. Biochim Biophys Acta 1724:367–374. doi: 10.1016/j.bbagen.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Wyckoff AC, Lockwood KL, Meyerett-Reid C, Michel BA, Bender H, Vercauteren KC, Zabel MD. 2013. Estimating prion adsorption capacity of soil by bioassay of subtracted infectivity from complex solutions (BASICS). PLoS One 8:e58630. doi: 10.1371/journal.pone.0058630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saunders SE, Bartz JC, Bartelt-Hunt SL. 2009. Prion protein adsorption to soil in a competitive matrix is slow and reduced. Environ Sci Technol 43:7728–7733. doi: 10.1021/es901385t. [DOI] [PubMed] [Google Scholar]
- 47.Saunders SE, Bartelt-Hunt SL, Bartz JC. 2008. Prions in the environment: occurrence, fate and mitigation. Prion 2:162–169. doi: 10.4161/pri.2.4.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saunders SE, Shikiya RA, Langenfeld K, Bartelt-Hunt SL, Bartz JC. 2011. Replication efficiency of soil-bound prions varies with soil type. J Virol 85:5476–5482. doi: 10.1128/JVI.00282-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnson CJ, Phillips KE, Schramm PT, McKenzie D, Aiken JM, Pedersen JA. 2006. Prions adhere to soil minerals and remain infectious. PLoS Pathog 2:e32. doi: 10.1371/journal.ppat.0020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuan Q, Eckland T, Telling G, Bartz J, Bartelt-Hunt S. 2015. Mitigation of prion infectivity and conversion capacity by a simulated natural process—repeated cycles of drying and wetting. PLoS Pathog 11:e1004638. doi: 10.1371/journal.ppat.1004638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hinckley GT, Johnson CJ, Jacobson KH, Bartholomay C, McMahon KD, McKenzie D, Aiken JM, Pedersen JA. 2008. Persistence of pathogenic prion protein during simulated wastewater treatment processes. Environ Sci Technol 42:5254–5259. doi: 10.1021/es703186e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson CJ, Bennett JP, Biro SM, Duque-Velasquez JC, Rodriguez CM, Bessen RA, Rocke TE. 2011. Degradation of the disease-associated prion protein by a serine protease from lichens. PLoS One 6:e19836. doi: 10.1371/journal.pone.0019836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu S, Reuter T, Gilroyed BH, Mitchell GB, Price LM, Dudas S, Braithwaite SL, Graham C, Czub S, Leonard JJ, Balachandran A, Neumann NF, Belosevic M, McAllister TA. 2014. Biodegradation of prions in compost. Environ Sci Technol 48:6909–6918. doi: 10.1021/es500916v. [DOI] [PubMed] [Google Scholar]
- 54.Johnson CJ, Pedersen JA, Chappell RJ, McKenzie D, Aiken JM. 2007. Oral transmissibility of prion disease is enhanced by binding to soil particles. PLoS Pathog 3:e93. doi: 10.1371/journal.ppat.0030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nichols TA, Spraker TR, Rigg TD, Meyerett-Reid C, Hoover C, Michel B, Bian J, Hoover E, Gidlewski T, Balachandran A, O'Rourke K, Telling GC, Bowen R, Zabel MD, Vercauteren KC. 2013. Intranasal inoculation of white-tailed deer (Odocoileus virginianus) with lyophilized chronic wasting disease prion particulate complexed to montmorillonite clay. PLoS One 8:e62455. doi: 10.1371/journal.pone.0062455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saunders SE, Bartelt-Hunt SL, Bartz JC. 2012. Resistance of soil-bound prions to rumen digestion. PLoS One 7:e44051. doi: 10.1371/journal.pone.0044051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maluquer de Motes C, Espinosa JC, Esteban A, Calvo M, Gironés R, Torres JM. 2012. Persistence of the bovine spongiform encephalopathy infectious agent in sewage. Environ Res 117:1–7. doi: 10.1016/j.envres.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 58.Maluquer de Motes C, Cano MJ, Torres JM, Pumarola M, Girones R. 2008. Detection and survival of prion agents in aquatic environments. Water Res 42:2465–2472. doi: 10.1016/j.watres.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 59.Miles SL, Takizawa K, Gerba CP, Pepper IL. 2011. Survival of infectious prions in water. J Environ Sci Health A Tox Hazard Subst Environ Eng 46:938–943. doi: 10.1080/10934529.2011.586247. [DOI] [PubMed] [Google Scholar]
- 60.Adamczyk B, Godlewski M, Zimny J, Zimny A. 2008. Wheat (Triticum aestivum) seedlings secrete proteases from the roots and, after protein addition, grow well on medium without inorganic nitrogen. Plant Biol 10:718–724. doi: 10.1111/j.1438-8677.2008.00079.x. [DOI] [PubMed] [Google Scholar]
- 61.White JF, Johnson H, Torres MS, Irizarry I. 2012. Nutritional endosymbiotic systems in plants: bacteria function like “quasi-organelles” to convert atmospheric nitrogen into plant nutrients. J Plant Pathol Microbiol 3:e104. doi: 10.4172/2157-7471.1000e104. [DOI] [Google Scholar]
- 62.Rasmussen J, Gilroyed BH, Reuter T, Badea A, Eudes F, Graf R, Laroche A, Kav NNV, McAllister TA. 2015. Protein can be taken up by damaged wheat roots and transported to the stem. J Plant Biol 58:1–7. doi: 10.1007/s12374-014-0258-z. [DOI] [Google Scholar]
- 63.Fürnkranz M, Lukesch B, Müller H, Huss H, Grube M, Berg G. 2012. Microbial diversity inside pumpkins: microhabitat-specific communities display a high antagonistic potential against phytopathogens. Microb Ecol 63:418–428. doi: 10.1007/s00248-011-9942-4. [DOI] [PubMed] [Google Scholar]
- 64.Bacon CW, White JF Jr, Stone JK. 2000. An overview of endophytic microbes: endophytism defined, p 3–30. In Bacon CW, White JF Jr (ed), Microbial endophytes. Marcel-Dekker, New York, NY. [Google Scholar]
- 65.Rasmussen J, Gilroyed BH, Reuter T, Dudas S, Neumann NF, Balachandran A, Kav NNV, Graham C, Czub S, McAllister TA. 2014. Can plants serve as a vector for prions causing chronic wasting disease? Prion 8:136–142. doi: 10.4161/pri.27963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Towne EG. 2000. Prairie vegetation and soil nutrient responses to ungulate carcasses. Oecologia 122:232–239. doi: 10.1007/PL00008851. [DOI] [PubMed] [Google Scholar]
- 67.Kufeld RC, Wallmo OC. 1973. Foods of the Rocky Mountain mule deer. Rocky Mountain Forest and Range Experiment Station, Forest Service, US Department of Agriculture, Fort Collins, CO. [Google Scholar]
- 68.Nichols TA, Fischer JW, Spraker TR, Kong Q, Vercauteren KC. 2015. CWD prions remain infectious after passage through the digestive system of coyotes (Canis latrans). Prion 9:367–375. doi: 10.1080/19336896.2015.1086061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miller MW, Swanson HM, Wolfe LL, Quartarone FG, Huwer SL, Southwick CH, Lukacs PM. 2008. Lions and prions and deer demise. PLoS One 3:e4019. doi: 10.1371/journal.pone.0004019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vercauteren KC, Pilon JL, Nash PB, Phillips GE, Fischer JW. 2012. Prion remains infectious after passage through digestive system of American crows (Corvus brachyrhynchos). PLoS One 7:e45774. doi: 10.1371/journal.pone.0045774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bian J, Khaychuk V, Angers RC, Fernandez-Borges N, Vidal E, Meyerett-Reid C, Kim S, Calvi CL, Bartz JC, Hoover EA, Agrimi U, Richt JA, Castilla J, Telling GC. 2017. Prion replication without host adaptation during interspecies transmissions. Proc Natl Acad Sci U S A 114:1141–1146. doi: 10.1073/pnas.1611891114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Daszak P, Cunningham AA, Hyatt AD. 2000. Emerging infectious diseases of wildlife—threats to biodiversity and human health. Science 287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- 73.Wasserberg G, Osnas EE, Rolley RE, Samuel MD. 2009. Host culling as an adaptive management tool for chronic wasting disease in white-tailed deer: a modelling study. J Appl Ecol 46:457–466. doi: 10.1111/j.1365-2664.2008.01576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Almberg ES, Cross PC, Johnson CJ, Heisey DM, Richards BJ. 2011. Modeling routes of chronic wasting disease transmission: environmental prion persistence promotes deer population decline and extinction. PLoS One 6:e19896. doi: 10.1371/journal.pone.0019896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miller MW, Hobbs NT, Tavener SJ. 2006. Dynamics of prion disease transmission in mule deer. Ecol Appl 16:2208–2214. doi: 10.1890/1051-0761(2006)016[2208:DOPDTI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 76.Dexter G, Tongue SC, Heasman L, Bellworthy SJ, Davis A, Moore SJ, Simmons MM, Sayers AR, Simmons HA, Matthews D. 2009. The evaluation of exposure risks for natural transmission of scrapie within an infected flock. BMC Vet Res 5:38. doi: 10.1186/1746-6148-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wyckoff AC, Galloway N, Meyerett-Reid C, Powers J, Spraker T, Monello RJ, Pulford B, Wild M, Antolin M, VerCauteren K, Zabel M. 2015. Prion amplification and hierarchical Bayesian modeling refine detection of prion infection. Sci Rep 5:8358. doi: 10.1038/srep08358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vasilyeva O, Oraby T, Lutscher F. 2015. Aggregation and environmental transmission in chronic wasting disease. Math Biosci Eng 12:209–231. doi: 10.3934/mbe.2015.12.209. [DOI] [PubMed] [Google Scholar]
- 79.Booth CJ, Johnson CJ, Pedersen JA. 2013. Microbial and enzymatic inactivation of prions in soil environments. Soil Biol Biochem 59:1–15. doi: 10.1016/j.soilbio.2012.12.016. [DOI] [Google Scholar]
- 80.Brown P, Rau EH, Lemieux P, Johnson BK, Bacote AE, Gajdusek DC. 2004. Infectivity studies of both ash and air emissions from simulated incineration of scrapie-contaminated tissues. Environ Sci Technol 38:6155–6160. doi: 10.1021/es040301z. [DOI] [PubMed] [Google Scholar]
- 81.Ding N, Neumann NF, Price LM, Braithwaite SL, Balachandran A, Mitchell G, Belosevic M, Gamal El-Din M. 2013. Kinetics of ozone inactivation of infectious prion protein. Appl Environ Microbiol 79:2721–2730. doi: 10.1128/AEM.03698-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ding N, Neumann NF, Price LM, Braithwaite SL, Balachandran A, Belosevic M, Gamal El-Din M. 2014. Ozone inactivation of infectious prions in rendering plant and municipal wastewaters. Sci Total Environ 470–471:717–725. doi: 10.1016/j.scitotenv.2013.09.099. [DOI] [PubMed] [Google Scholar]
- 83.Goñi F, Knudsen E, Schreiber F, Scholtzova H, Panckiwewicz J, Carp R, Meeker H, Rubenstein R, Brown D, Sy M. 2005. Mucosal vaccination delays or prevents prion infection via an oral route. Neuroscience 133:413–421. doi: 10.1016/j.neuroscience.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 84.Goñi F, Mathiason CK, Yim L, Wong K, Hayes-Klug J, Nalls A, Peyser D, Estevez V, Denkers N, Xu J, Osborn DA, Miller KV, Warren RJ, Brown DR, Chabalgoity JA, Hoover EA, Wisniewski T. 2015. Mucosal immunization with an attenuated Salmonella vaccine partially protects white-tailed deer from chronic wasting disease. Vaccine 33:726–733. doi: 10.1016/j.vaccine.2014.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]