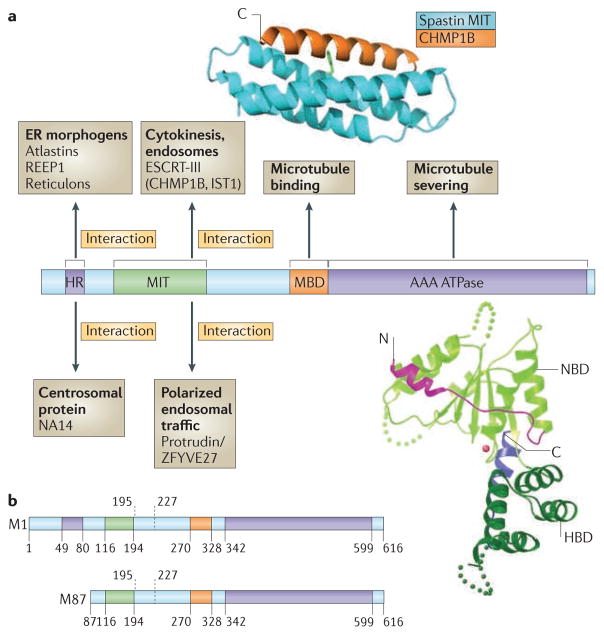
Figure 1. Spastin domain structure and interacting proteins.
Domains in the spastin protein. a | The hydrophobic region (HR) possibly forms an intramembrane hairpin loop. The microtubule interacting and trafficking domain (MIT) forms a three-helix bundle that interacts with a helix in the endosomal sorting complex required for transport III (ESCRT-III) proteins charged multivesicular body protein 1B (CHMP1B) and IST1. The microtubule binding domain (MBD) is necessary for spastin to bind to microtubules and is required for microtubule severing. The AAA (ATPases associated with diverse cellular activities) ATPase domain contains the enzymatic activity of the protein that is essential for microtubule breakage. The regions to which interaction sites with known binding partners have been narrowed are indicated. The structure of the interaction between the spastin MIT domain and CHMP1B is shown, as is the structure of the Drosophila AAA ATPase domain. The amino (N)- and carboxy (C)-terminal helices are shown in magenta and blue, respectively. NBD, nucleotide binding domain; HBD, helix bundle domain; REEP1, receptor expression-enhancing protein 1. The spastin structure and interaction with CHMP1B are reproduced, with permission, from REF. 30 © (2008) Macmillan Publishers Ltd. All rights reserved. The structure of the Drosophila AAA ATPase domain is reproduced, with permission, from REF. 18 © (2008) Macmillan Publishers Ltd. All right reserved. b | The domain structure of the M1 spastin isoform and the M87 spastin isoform, with amino-acid numbers indicated. The position of the alternatively spliced exon 4 is shown by the dashed lines.