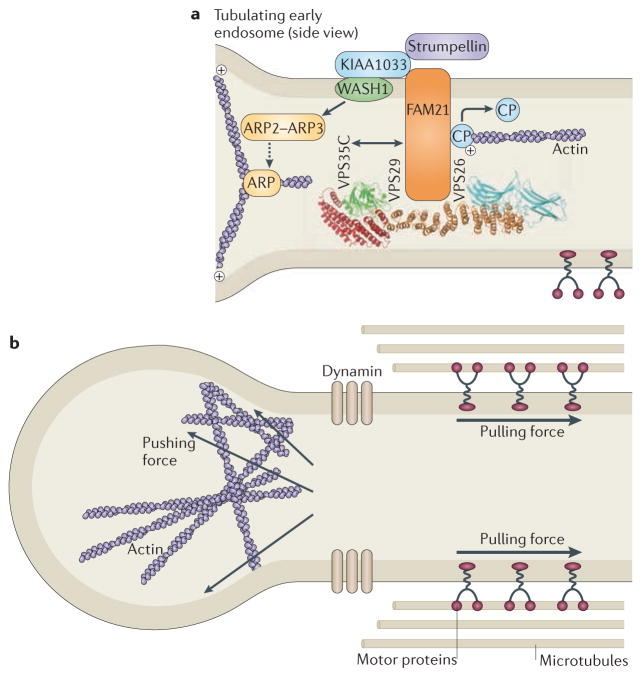
Figure 3. The strumpellin–WAsH complex.
a | The strumpellin–WASH (Wiskott–Aldrich syndrome protein and SCAR homologue) complex is recruited to endosomes by interaction between family with sequence similarity 21 (FAM21) and the vacuolar protein sorting-associated protein 35 (VPS35) component of the VPS26–VPS29–VPS35 cargo-selective retromer. Strumpellin probably interacts with the WASH complex via KIAA1033, but this remains to be proven. WASH interacts with, and activates, the actin-related protein 2 (ARP2)–ARP3 complex, which nucleates the formation of branched actin filaments. FAM21 has been proposed to interact with capping protein (CP) and promote its removal from the actin plus end, thus enhancing actin polymerization. b | In the absence of components of the WASH complex, early endosomal tubulation is enhanced, leading to the suggestion that the actin network generated by the complex is required to generate a pushing force on the tubule, which, combined with a pulling force generated by microtubule-based motors, promotes fission of the tubule by dynamin. The structure of the VPS26–VPS29–VPS35 complex is reproduced, with permission, from REF. 100 © (2007) Macmillan Publishers Ltd. All rights reserved.