Figure 4. Model of NIPA1 action on bone morphogenetic protein receptor type-2 (BMPRII) traffic.
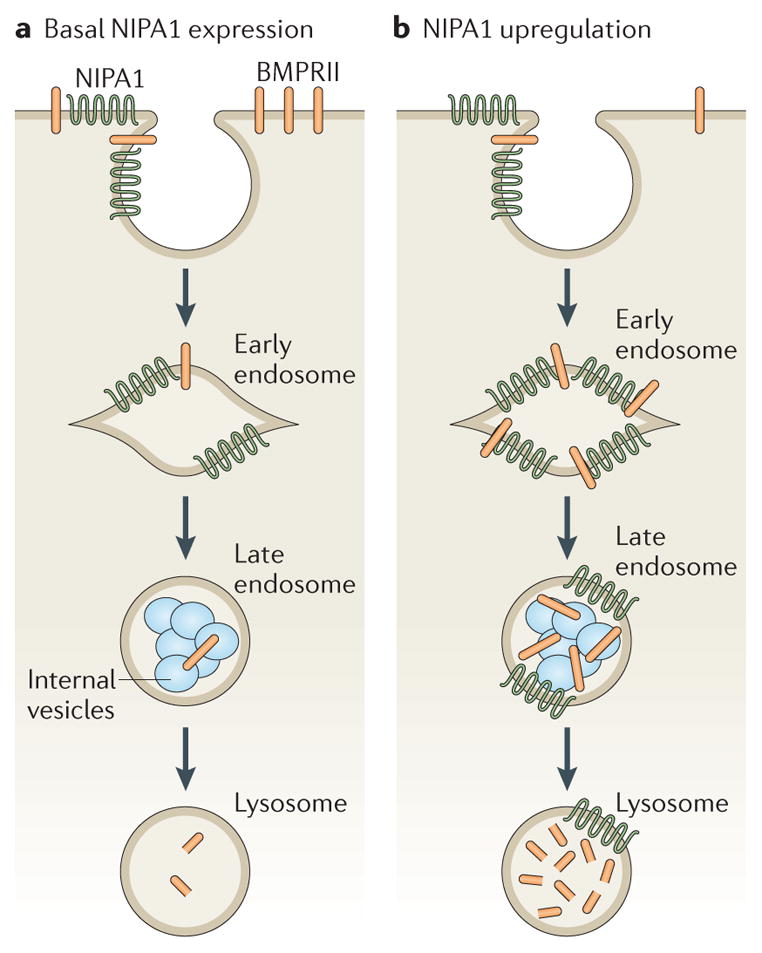
a | In cells that express normal levels of non imprinted in Prader-Willi/Angelman syndrome 1 (NIPA1), most bone morphogenetic protein receptor type-2 (BMPRII) is situated on the plasma membrane, with small amounts in early and recycling endosomal compartments. NIPA1 is found in endosomal compartments and at the plasma membrane. Lysosomal inhibition and cellular depletion of NIPA1 both increase cellular BMPRII levels, suggesting that NIPA1 promotes degradation of BMPRII in the lysosome. b | Consistent with this idea, overexpression of NIPA1 causes dramatic internalization of BMPRII into endosomal and lysosomal compartments, accompanied by increased lysosomal degradation of BMPRII. Certain features in these diagrams are conjectural — for example, it is not known whether the initial interaction between NIPA1 and BMPRII occurs at the plasma membrane or endosomes, whether NIPA1 is degraded in lysosomes or whether it can recycle back to the plasma membrane.
