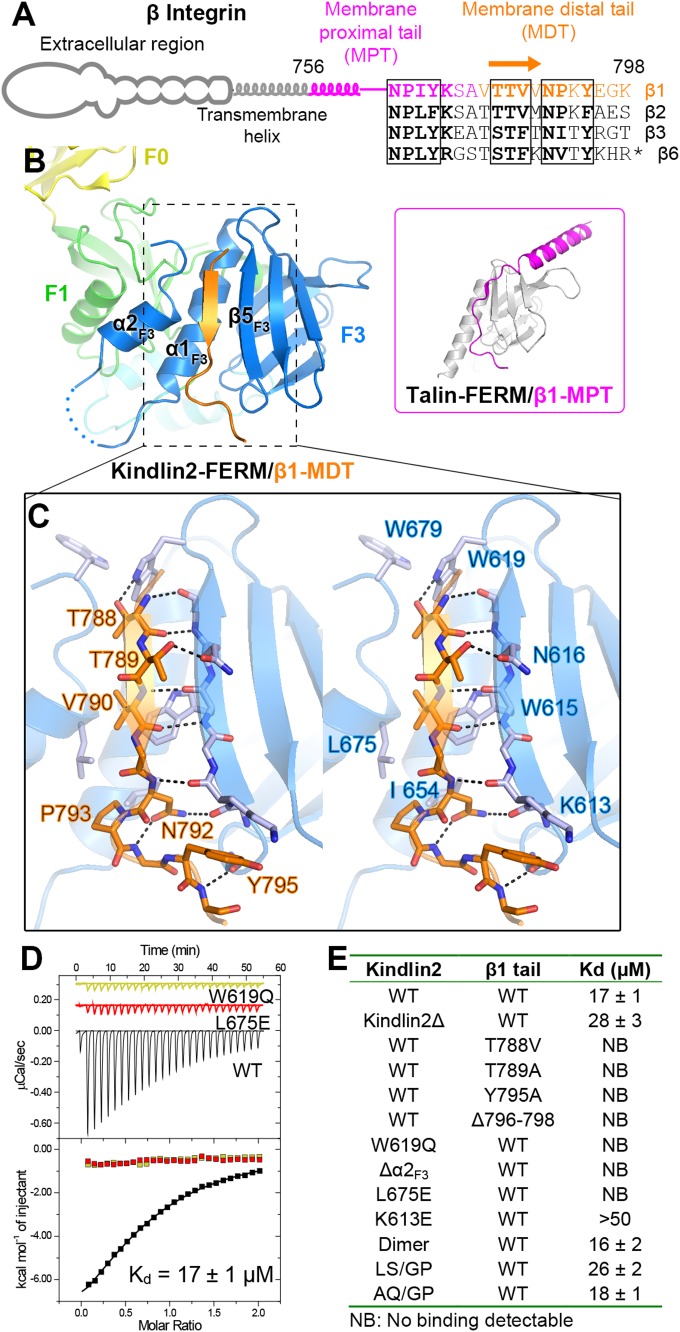
Fig. 2.
Structural and biochemical characterization of the kindlin2/β1-tail interaction. (A) The cartoon diagram of β-integrins. The sequences of the cytoplasmic tails from mouse β-integrins are aligned. The C-terminal 11 residues in the β6-tail are omitted from the alignment. The two NxPY motifs and the TTV motif are boxed. (B) The binding of the β1-MDT to kindlin2-F3. The complex structure of talin-FERM/β1-MPT (PDB ID: 3G9W) is shown in the Inset for comparison. (C) Molecular details of the β1-MDT/F3 interaction (stereoview). H-bonds are indicated by dashed lines. (D) ITC curves showing the interaction between the β1-tail and the kindlin2 proteins (wild-type as well as two TTV-binding deficient mutants). (E) The dissociation constants of the binding reactions of various forms of kindlin2 and the β1-tail (thioredoxin-tagged) derived from the ITC-based assays.