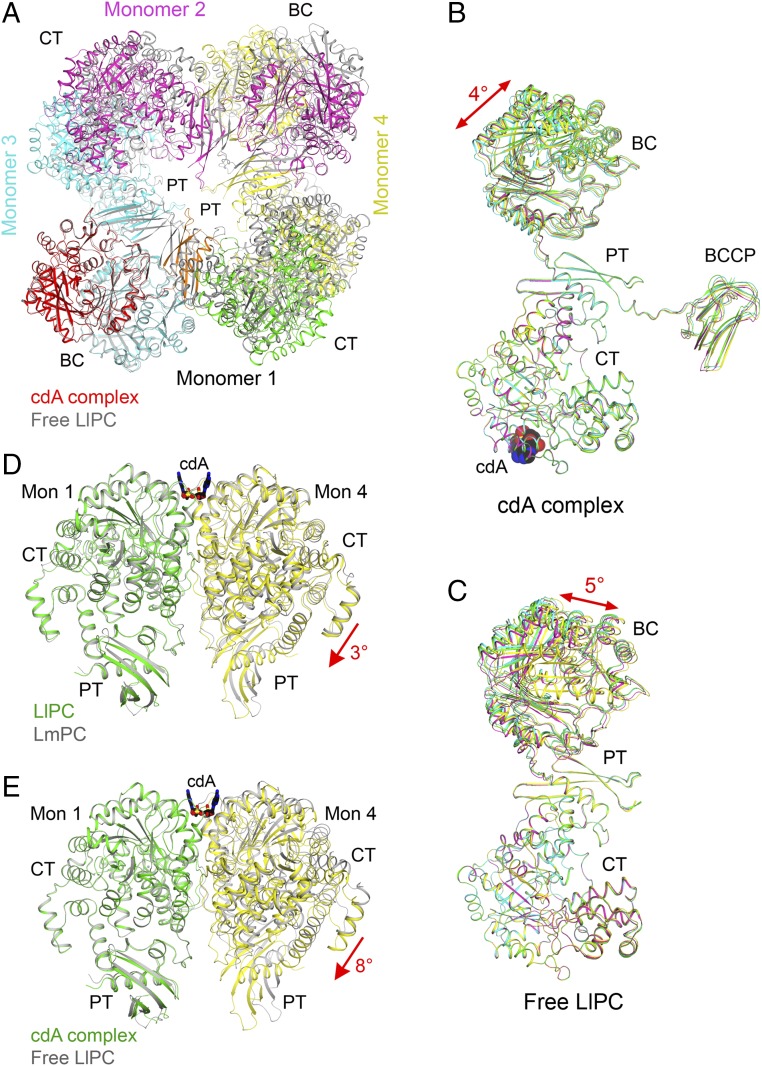
Fig. 4.
Large conformational changes in LlPC upon c-di-AMP binding. (A) Overlay of the structures of the c-di-AMP complex (in color) and free enzyme (gray) of LlPC tetramer. The BC dimer of monomers 1 and 3 was used for the overlay. The BCCP domains in the c-di-AMP complex were removed for clarity. (B) Overlay of the four monomers in the c-di-AMP complex of LlPC by their CT domains. A small difference is observed for the BC and BCCP domains. (C) Overlay of the four monomers in the free enzyme of LlPC by their CT domains. The four monomers have roughly the same conformation as well. (D) Overlay of the LlPC CT dimer in the c-di-AMP complex (in color) with the LmPC CT dimer in the c-di-AMP complex (gray). The CT domain in monomer 1 was used for the overlay, but the overall structures of the two dimers are similar, with rms distance of 0.89 Å for their equivalent Cα atoms. (E) Overlay of the LlPC CT dimer in the c-di-AMP complex (in color) with that in the free enzyme (gray). A large difference for the CT domain in monomer 4 is seen, corresponding to a 5° rotation (red arrow).