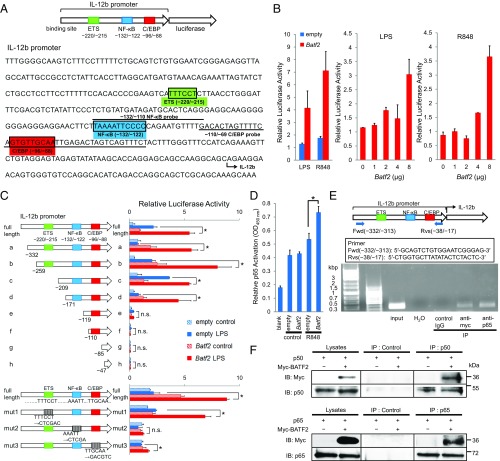
Fig. 5.
Analysis of the mechanism responsible for the effect of BATF2 on IL-12 p40 expression. (A) A schematic of the luciferase reporter construct with the mouse IL-12 p40 promoter and its sequence are shown. Underlined sequences indicate the locations of oligonucleotide probes used in DNA-binding assays and EMSAs. Potential transcription factor binding consensus sequences mentioned in the text are boxed and labeled. (B) The mouse IL-12 p40 promoter luciferase reporter construct was transfected into RAW 264.7 cells along with a Batf2 construct. Cells were activated with LPS or R848 at 24 h posttransfection, and luciferase activity was measured 24 h after stimulation (Left). The mouse IL-12 p40 promoter luciferase reporter construct was cotransfected with increasing amounts of Batf2 construct, and cells were activated with LPS (Middle) or R848 (Right). Luciferase activities relative to empty vector control are shown as a representative mean ± SEM. (C) Deletion-mutant (Upper) and substitution-mutant (Lower) analyses of the IL-12 p40 promoter. Mutants were transfected into RAW 264.7 cells with a Batf2 or empty construct. Twenty-four hours after transfection, cells were activated with LPS for 24 h and compared with nonactivated control cells. The result shown for each mutant represents the mean ± SEM of data from three independent experiments. (D) DNA-binding activities of p65 in RAW 264.7 cells transfected with Batf2 or empty constructs were measured with a TransAM Flexi NF-κB Transcription Factor Assay Kit. (E) ChIP analysis was performed using RAW 264.7 cells overexpressing Myc-tagged Batf2. Immunoprecipitation was performed using the following antibodies: normal rabbit IgG (control IgG) and anti–c-Myc or anti–NF-κB p65. Sample DNA was analyzed by PCR using primers that detect sequences containing the Il12b promoter. (F) Data from coimmunoprecipitation experiments assessing BATF2 binding to p50 and p65. For the p50 analysis, cell extracts from RAW 264.7 cells overexpressing both Nfkb1 (p50) and Myc-tagged Batf2 were prepared. After immunoprecipitation with anti-p50 antibody or normal IgG (Control), samples were analyzed with anti–c-Myc antibody by Western blotting. The interactions between Myc-tagged BATF2 and p65 were analyzed similarly. Data are representative of at least two independent experiments. In all figures, *P < 0.05; n.s., not significant (P > 0.05).