Abstract
Although mutations in the GJB2 gene sequence make up the majority of variants causing autosomal-recessive non-syndromic hearing loss, few large deletions have been shown to contribute to DFNB1 deafness. Currently, genetic testing for DFNB1 hearing loss includes GJB2 sequencing and DFNB1 deletion analysis for two common large deletions, del(GJB6-D13S1830) and del(GJB6-D13S1854). Here, we report frequency in Russia, clinical significance and evolutionary origins of a 101 kb deletion, del(GJB2-D13S175), recently identified by us. In multiethnic cohort of 1104 unrelated hearing loss patients with biallelic mutations at the DFNB1 locus, the del(GJB2-D13S175) allele frequency of up to 0.5% (11/2208) was determined and this allele was shown to be predominantly associated with profound sensorineural hearing loss. Additionally, eight previously unpublished GJB2 mutations were described in this study. All patients carrying del(GJB2-D13S175) were of the Ingush ancestry. Among normal hearing individuals, del(GJB2-D13S175) was observed in Russian Republic of Ingushetia with a carrier rate of ~1% (2/241). Analysis of haplotypes associated with the deletion revealed a common founder in the Ingushes, with age of the deletion being ~3000 years old. Since del(GJB2-D13S175) was missed by standard methods of GJB2 analysis, del(GJB2-D13S175) detection has been added to our routine testing strategy for DFNB1 hearing loss.
Introduction
Pathogenic variants in the DFNB1 locus (MIM#220290) are the most commonly identified cause of congenital, recessively inherited, sensorineural non-syndromic hearing loss. This locus contains the GJB2 gene, encoding the connexin 26 protein. The mutation spectrum of GJB2 varies in different populations. There are ethnic-specific frequent mutations in the GJB2 gene as a result of the founder effect.1 Although, more than 300 mutations in the GJB2 gene sequence have been described and make up the majority of causative variants, six large deletions have been shown to contribute to DFNB1 deafness (The Human Gene Mutation Database or HGMD, Professional 2016.2). DFNB1 patients carry a large deletion in trans with a GJB2 variant or they are homozygous (or double heterozygous) for the large deletion(s).2, 3, 4, 5, 6, 7, 8, 9, 10
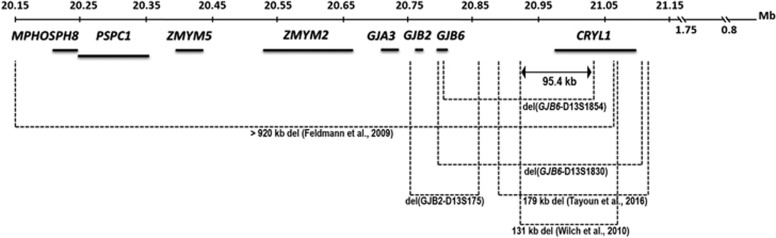
Two mechanisms of pathogenesis for the large DFNB1 deletions have been hypothesized. Four large deletions lie upstream of GJB2 and do not affect GJB2, but were shown to reduce GJB2 expression by disrupting unidentified cis-regulatory element. Presumably, cis regulatory element is located within the common 95.4 kb genomic interval that is deleted in all four mutant alleles (Figure 1).7, 11, 12, 13, 14 This interval encompasses the upstream CRYL1 gene and extends telomerically. The most common deletions being 309 and 232 kb, del(GJB6-D13S1830) and del(GJB6-D13S1854), respectively, truncate the upstream GJB6 and CRYL1 genes. Two private DFNB1 deletions of 131 and 179 kb involve CRYL1 only.7, 9 Two additional mutations directly affect the GJB2 gene by deletion of the entire GJB2 sequence. One of them is 920 kb in size involves seven more other genes and was found in only one deaf individual.7 The second deletion of 101 kb, del(GJB2-D13S175), encompassing GJB2 and GJB6, which we described recently, has been identified in three unrelated Russian patients.10
Figure 1.
Schematic map of the six large deletions described at DFNB1 locus on chromosome 13q11-12. The DNA section encompasses eight genes (bold horizontal lines), the breakpoints of the deletions (dotted brackets). All elements are drawn approximately to scale. The del(GJB2-D13S175) mutation was investigated in this study. All genomic coordinates are based on Human Genome Build GRCh37.p13/hg19.
Currently, clinical DFNB1 testing for non-syndromic hearing loss patients includes GJB2 sequencing and DFNB1 deletion analysis for only the two common deletions del(GJB6-D13S1830) and del(GJB6-D13S1854). These deletions account for 1–10% of all the DFNB1 alleles in most populations in Europe, America and Australia, but the del(GJB6-D13S1830) mutation is much more frequent than del(GJB6-D13S1854).5, 6, 15, 16, 17 All the studied chromosomes carrying the del(GJB6-D13S1854) mutation share a common founder, while two founders for del(GJB6-D13S1830) have been demonstrated.5, 6
The frequency of del(GJB6-D13S1830) observed among Russian patients is 0.3% of the DFNB1-mutant alleles, whereas del(GJB6-D13S1854) has not been found.18, 19, 20, 21 Previously, we have published the spectrum of GJB2/DFNB1 in 705 Russian patients, but this study does not include the testing for recently found mutation del(GJB2-D13S175).19 Here, update findings of investigation of a larger cohort of hearing loss patients from Russia are present. We report the high frequency, clinical significance and the evolutionary origins of del(GJB2-D13S175). Additionally, eight novel GJB2 mutations were found.
Materials and methods
Study cohort and population samples
We analyzed genomic DNA samples from 2569 unrelated individuals with non-syndromic sensoneural hearing loss that were referred for DNA analysis of GJB2/DFNB1 at the Laboratory of DNA Diagnostics, Research Centre for Medical Genetics, Moscow, between years 2000 and 2015, including the patients from cohort that has previously been reported.19 DNA analysis of GJB2 was performed on all patients as previously described.19 Briefly, this is a two-step protocol starting with the common point mutations test, followed by exon 2 sequencing analysis in the cases when one or no common mutation has been revealed. Then samples with one heterozygous mutation were tested for del(GJB6-D13S1830). The common mutation test lay in two reactions, a multiplex PCR with primer pairs for five deletions (c.35delG, c.313_326del14, c.235delC, c.167delT and c.358_360delGAG) and restriction fragment length polymorphism (RLFP) analysis of c.−23+1G>A, followed by gel-based fragment analysis to differentiate wild-type and mutation alleles. The nomenclature of all GJB2 sequence variants was based on complimentary DNA reference sequence of the NM_004004.5 transcript.
The study cohort consisted of 1101 patients carrying biallelic mutations in the GJB2 gene, 1465 individuals who carry one or no pathogenic GJB2 variant, 3 patients in which the PCR product was not amplified with GJB2- and D13S175-specific primers. One patient ‘homozygous’ for c.35delG, but whose father had no GJB2 mutations and two of three patients without sequences of the GJB2 and D13S175 were described in previous article about the del(GJB2-D13S175) identification.10 Among patients with homozygous GJB2 mutation, there were 691 patients whose parents’ DNA were not available.
The population samples have been selected from the Biobank of North Eurasia.22 The biobank’s samples were collected in 1998–2011 during the extensive field trips to the rural areas. The samples analyzed in this study came from the two regions of Russia: Republic of Ingushetia and Chechen Republic. The sampled persons represented the corresponding indigenous rural populations up to the third generation, that is, all four grandparents belonged to the given ethnic groups and were born within the given region. The population samples included 241 persons from Ingushetia Republic (151 self-identified Ingushes and 90 self-identified Chechens), and 147 self-identified Chechens from Chechen Republic.
All written informed consent forms signed by the participants or the guardians of the under age participants involved in our study were obtained before the testing procedures. This study was approved by the local Ethics Committee of Federal State Budgetary Institution ‘Research Centre for Medical Genetics’, Moscow, Russia.
del(GJB2-D13S175) deletion test
The del(GJB2-D13S175) deletion test was performed using diplex PCR with deletion-specific breakpoint primers (F—5′-GCTCTGCCCAGATGAAGATCTC-3′, R—5′-CCTTCCAGGAGAGTTCACAACTC-3′) and GJB2 exon 2 specific primer pair to amplify a PCR control fragment (F—5′-GTGATTCCTGTGTTGTGTGCATTC-3′, R—5′-CCTCATCCCTCTCATGCTGTC-3′). The amplified products were separated by polyacrylamide gel electrophoresis with subsequent ethidium bromide stain and ultraviolet visualization.
STRs analysis and del(GJB2-D13S175) dating
Three short tandem repeats (STRs) flanking del(GJB2-D13S175) were genotyped by PCR amplification and size analyses of the amplified products by polyacrylamide gel electrophoresis. The STRs and the primers used for amplification are following: D13S1316F—5′-GATTGCACCACTACATACCAGC-3′, D13S1316R—5′-CTTTGACTCTCCATGCTGCATTC-3′, D13S1275F—5′-GCTAGTCTTCAGATTACCTTAGAATATACC-3′, D13S1275R—5′-CAGCATGAACTTTACCAGAATTCC-3′, D13S232F—5′-GCTCACTGCTCTTGTGATTCTG-3′, D13S232R—5′-GGCACAGAAATAAATGTTGATGTATGTAC-3′. The STR allele designation corresponds to appropriate repeat number, which was fixed by the control DNA samples, previously sequenced by using the ABI Dye Terminator, version 1 and run on a 3130 ABI genetic analyzer (Applied Biosystems, Tokyo, Japan). The STR genotyping was performed in 8 patients with del(GJB2-D13S175), in 6 their parents and in 90 healthy Ingush individuals. Haplotypes were imputed from family analysis data. To estimate the age of del(GJB2-D13S175), we followed the approach that have been offered and previously proven.23, 24, 25, 26 STATISTICA 10 (StatSoft. Inc. (2011), Tulsa, OK, USA) was used for other calculations.
Results
A novel GJB2 mutation
A total of 39 pathogenic or likely pathogenic variants in DFNB1 locus were found in Russian cohort of patients with non-syndromic sensoneural hearing loss, including previously published19 and novel samples collected between year 2000 and 2015 in the Laboratory of DNA Diagnostics. In Table 1, an allele frequency of these variants and references are present. Among the identified variants, eight mutations have for the first time been described in this work. These are five likely pathogenic missense substitutions, two pathogenic nonsense substitutions and one pathogenic frame shifting deletion according to the ACMG guidelines for classifying pathogenic variants27 (Table 2).
Table 1. DFNB1 alleles in Russian patients.
| Mutation | Effect | % (number) of alleles among 2208 chrs. | Referencea |
|---|---|---|---|
| c.35delG | p.Gly12Valfs*2 | 77.0 (1700) | CD972240 |
| c.313_326del14 | p.Lys105Glyfs*5 | 4.7 (103) | CD991732 |
| c.−23+1G>A | IVS1+1G>A | 4.4 (97) | CS991407 |
| c.101T>C | p.Met34Thr | 2.9 (65) | CM970679 |
| c.235delC | p.Leu79Cysfs*3 | 2.3 (51) | CD991730 |
| c.167delT | p.Leu56Argfs*25 | 1.5 (33) | CD972241 |
| c.109G>A | p.Val37Ile | 1.2 (27) | CM000016 |
| c.269T>C | p.Leu90Pro | 1.0 (23) | CM990691 |
| c.358_360delGAG | p.Glu120del | 0.9 (19) | CD993053 |
| c.290dupA | p.Tyr97* | 0.7 (15) | CI014787 |
| del(GJB2-D13S175) | GJB2-GJB6 deletion | 0.5 (11) | CG145665 |
| c.551G>C | p.Arg184Pro | 0.5 (10) | CM992895 |
| del(GJB6-D13S1830) | Putative abnormal regulation | 0.3 (6) | CG024899 |
| c.71G>A | p.Trp24* | 0.3 (6) | CM970678 |
| c.380G>A | p.Arg127His | 0.2 (5) | CM980930 |
| c.95G>A | p.Arg32His | 0.2 (4) | CM013721 |
| c.427C>T | p.Arg143Trp | 0.2 (4) | CM000018 |
| c.119C>A | p.Ala40Glu | 0.1 (2) | CM041349 |
| c.139G>T | p.Glu47* | 0.1 (2) | CM970680 |
| c.266T>C | p.Leu89Pro | 0.1 (2) | This report |
| c.334_335delAA | p.Lys112Glufs*2 | 0.1 (2) | CD982678 |
| c.559_561delGAG | p.Glu187del | 0.1 (2) | CD042866 |
| c.598G>A | p.Gly200Arg | 0.1 (2) | CM0910079 |
| c.632_633delGT | p.Cys211Leufs*5 | 0.1 (2) | CD982679 |
| c.31_68del38 | p.Gly11Leufs*24 | <0.1 (1) | CG973465 |
| c.94C>G | p.Arg32Gly | <0.1 (1) | This report |
| c.129delG | p.Trp44Glyfs*38 | <0.1 (1) | Bliznetz et al.b |
| c.205T>C | p.Phe69Leu | <0.1 (1) | This report |
| c.245T>A | p.Ile82Asn | <0.1 (1) | This report |
| c.246C>G | p.Ile82Met | <0.1 (1) | CM021271 |
| c.257C>G | p.Thr86Arg | <0.1 (1) | CM031189 |
| c.385G>A | p.Glu129Lys | <0.1 (1) | CM014194 |
| c.399G>A | p.Trp133* | <0.1 (1) | This reportc |
| c.402G>A | p.Trp134* | <0.1 (1) | This report |
| c.419T>G | p.Ile140Ser | <0.1 (1) | CM053901 |
| c.502_601del10 | p.Lys168Profs*5 | <0.1 (1) | This report |
| c.532G>A | p.Val178Met | <0.1 (1) | This report |
| c.550C>T | p.Arg184Trp | <0.1 (1) | CM000709 |
| c.614T>C | p.Leu205Pro | <0.1 (1) | CM055278 |
A number following CD, CS, CM, CI or CG is the HGMD Professional 2016.2 accession number for the mutation.
Patient with the novel c.129delG allele is the same with that previously published.19
The mutation c.399G>A has NCBI dbSNP accession number rs777225786, but for it, a population frequency and clinical significance are unknown and observation in hearing loss individuals is not described. The names of the novel mutations are highlight in bold type.
Table 2. Characteristics of the novel variants in the GJB2 gene.
| Variant | Protein effect | Affected protein domain | Pathogenicitya | Pathogenic criteriaa | Genotype, number of family members with genotype | Severity of hearing loss | Ethnicity |
|---|---|---|---|---|---|---|---|
| c.94C>G | Missense | TM1 | Likely pathogenic | PM2,3,5+PP1,2,3 | c.[35delG]+[94C>G], 2 | Moderately severe, severe | Tatar |
| c.205T>C | Missense | EC1 | Likely pathogenic | PM2,3+PP2,3 | c.[35delG]+[205T>C], 1 | Severe | Unknown |
| c.245T>A | Missense | TM2 | Likely pathogenic | PM2,3,5+PP2,3 | c.[35delG]+[245T>A], 1 | Severe | Tatar/Russian |
| c.266T>C | Missense | TM2 | Likely pathogenic | PM2,3+PP1,2,3 | c.[35delG]+[266T>C], 2 | Severe | Russian |
| c.[109G>A]+[266T>C], 1 | Severe to profound | Russian | |||||
| c.399G>A | Nonsense | TM3 | Pathogenic | PVS1+PS1+PM2+PP3 | c.[35delG]+[399G>A], 1 | Unknown | Russian |
| c.402G>A | Nonsense | TM3 | Pathogenic | PVS1+PS1+PM2,3,5+PP3 | c.[35delG]+[402G>A], 1 | Severe | Tajik |
| c.502_601del10 | Frameshift | EC2 | Pathogenic | PVS1+PM2,3+PP3 | c.[35delG]+[502_601del10], 1 | Severe | Russian |
| c.532G>A | Missense | EC2 | Likely pathogenic | PM2,3,5+PP2,3 | c.[35delG]+[457G>A,532G>A]b, 1 | Severe to profound | Russian |
The novel GJB2 mutations have not been detected in 1465 individuals who carry one or no pathogenic/likely pathogenic GJB2 variant and have not been registered as at January 2017 in the databases of 1000 Genomes Project (http://browser.1000genomes.org, http://www.ensembl.org), ExAC (http://exac.broadinstitute.org), NCBI dbSNP and HGMD Professional 2016.2. An exception is mutation c.399G>A having NCBI dbSNP accession number rs777225786, of which population frequency and clinical significance are unknown and observation in hearing loss individuals is not described. The use of the prediction programs has shown that all novel mutations are ‘disease causing’ by software Mutation testing (http://www.mutationtaster.org) and all five missense substitutions are ‘deleterious/damaging’ by PROVEAN, SIFT and PolyPhen-2 (http://provean.jcvi.org, http://genetics.bwh.harvard.edu/pph2). In this work, Sanger sequencing of the PCR product, which covers the entire GJB2 coding region, was performed using one primer pair. Consequently, when a nucleotide substitution in combination with a deletion (or an insertion) in the coding region was observed in a compound heterozygous genotype, both of these variants were presented together in a single forward sequence chromatogram and in a single reverse sequence chromatogram. Therefore, analysis of both sequence chromatograms, along with testing parental samples, was performed to determine whether the variant occurs in cis or in trans. Additional information about novel mutations, including family history and DNA sequence chromatograms, is presented in Supplementary Figure S1.
Frequency of del(GJB2-D13S175) among the DFNB1 alleles in Russia
Previously, we documented the novel 101 kb deletion, del(GJB2-D13S175), in three unrelated Russian patients with sensorineural hearing loss and identified its breakpoints (NC_000013.10: g.20 757 021_20 858 394del). This deletion encompasses the entire GJB2 and GJB6 genes, and so it has been observed to cause a false homozygosity of the GJB2 c.35delG mutation in one compound heterozygous patient and the lack of the GJB2 gene sequence in two patients homozygous for the large deletion.10 Thus, an apparent homozygosity of the GJB2 mutation can conceal a hemizygosity, if samples are not available from the patient’s parents, and segregation analysis cannot be performed. Since most of our hearing loss patients have been investigated without material from parents, we proposed that among patients homozygous for the GJB2 mutation, there are carriers of del(GJB2-D13S175).
Four (0.6%) carriers of del(GJB2-D13S175) have been discovered from the sample analysis of 691 patients, in whom previous testing at our laboratory detected ‘homozygous’ GJB2 pathogenic variant but their parents’ DNA were not tested. Among them, two patients were GJB2 heterozygous for the c.35delG variant and the remaining two patients carried one of the c.290dupA or c.−23+1G>A variants (Table 3). Later, parental DNA has been tested to confirm trans configuration for the del(GJB2-D13S175) and c.290dupA alleles, while DNA from three patient’s parents was not available. One more patient homozygous for del(GJB2-D13S175) was revealed using deletion-specific breakpoint primers, after PCR amplification with GJB2- and D13S175-specific primers was observed to be absent, in addition to two homozygous and one compound heterozygous for the deletion patients described previously.10 Thus, a total of 11 deletion-carrying chromosomes were detected in 8 unrelated patients (Table 3) that makes 0.5% of all DFNB1 alleles in a study cohort of 1104 patients, carrying biallelic mutations in GJB2/DFNB1. In Table 1, a del(GJB2-D13S175) position from a list of all DFNB1 mutations in the study cohort is present.
Table 3. Characteristics of patients with the del(GJB2-D13S175) mutation.
| Patient | Pathological genotype | Ethnicity (birth place) | Procedure and age at first hearing testing | Severity of hearing loss | Age at cochlear implantation | Outcome |
|---|---|---|---|---|---|---|
| A | del(GJB2-D13S175)/c.35delG | Ingush (Ingushetia) | CPA, 4 y | Moderate | Hearing aids were used | Good |
| B | del(GJB2-D13S175)/c.−23+1G>A | Ingush (Ingushetia) | OAE at birth, ABR, 12 m | Profound | 31 months | Good |
| C | del(GJB2-D13S175)/c.35delG | Ingush (Ingushetia) | OAE at birth, ABR, 7 m | Profound | 12 months | Good |
| D | del(GJB2-D13S175)/c.290dupA | Ingush (Ingushetia) | OAE at birth, ABR, 14 m | Profound | 21 months | Good |
| E | del(GJB2-D13S175)/del(GJB2-D13S175) | Ingush (Ingushetia) | OAE at birth, ABR, 16 m | Profound | 27 months | Good |
| F | del(GJB2-D13S175)/del(GJB2-D13S175) | Ingush (Ingushetia) | OAE at birth, ABR, 16 m | Profound | 36 months | Good |
| G | del(GJB2-D13S175)/c.35delG | Ingush/Russian (Moscow) | OAE at birth, ABR, 5 m | Profound | 11 months | Good |
| H | del(GJB2-D13S175)/del(GJB2-D13S175) | Ingush (Chechnya) | OAE at birth | Unknown | Unknown | Unknown |
Abbreviations: ABR, auditory brainstem response audiometry; CPA, conditioned play audiometry; OAE, evoked otoacoustic emissions. By ‘good outcome’, we mean improvements in auditory skills as well as in the development of speech production.
Clinical follow-up of the del(GJB2-D13S175)-carrying patients
All patients with the del(GJB2-D13S175) deletion were diagnosed as having bilateral sensorineural hearing loss (Table 3). Hearing impairment in patient A was identified first at the age of 4 years using conditioned play audiometry. Pure-tone audiometry between 4 and 10 years of age demonstrated bilateral moderate sensorineural hearing loss in this patient (Supplementary Figure S2(1)). Hearing loss in patients B, C, D, E, F, G and H was identified during newborn hearing screening performed using transient-evoked otoacoustic emission and distortion-product otoacoustic emission, evoked otoacoustic emission from the patients was absent (Supplementary Figures S2(2), S2(3)). Additionally, patients B, C, D, E, F and G audiological examination included auditory brainstem response audiometry, which revealed bilateral profound deafness in these patients since auditory brainstem response audiometries were absent at 70, 90 and 100 dBnHL (Supplementary Figure S2(4)). Middle ear endoscopy, tympanometry and X-ray computed tomography of the temporal bone did not detect any middle or inner ear malformation in patients A, B, C, D, E, F and G. Audiological testing data from patient H were not available.
Patients A, B, C, D, E, F and G underwent medical genetic counseling and pediatrician, neurologists, ophthalmologist and dermatologist examination. Only data from medical genetic evaluation of patient H were available. Patient A was diagnosed with psoriasis and has a twin brother with the same severity of hearing loss and satisfying outcomes were derived from the use of hearing aids. Astigmatism and a quite dry skin was revealed in patient F.
Evolutionary origins and heterozygous carriage of del(GJB2-D13S175)
Seven patients with del(GJB2-D13S175) and their parents were the Ingushes. The patient G was of mixed ancestry (Table 3). He has inherited the deletion from his father who had heterozygous del(GJB2-D13S175) allele and whose father, in turn, was the Ingush. The Ingushes are a Caucasian native ethnic group of the North Caucasus, mostly inhabiting Russian Republic of Ingushetia. According to the 2010 Russian Census, Ingushes make up 94.1% of republic's population and 0.3% of Russia's population. The second ethnic group of Ingushetia includes Chechens (4.6%), genetically close to Ingushes. Chechens make up 95.3% of population of the Chechen Republic, geographically neighboring with Ingushetia.
We proposed a high frequency of del(GJB2-D13S175) in Ingush and Chechen populations, and investigated the population carrier frequency of del(GJB2-D13S175) and common GJB2 mutations (c.35delG, c.313_326del14, c.235delC and c.358_360delGAG) among 151 Ingushes from Ingushetia, 90 native Chechens from Ingushetia and 147 Chechens from Chechnya, who had a normal hearing. The results of this investigation are presented in Table 4. The del(GJB2-D13S175) mutation was found in two individuals from Ingushetia while it was not observed among Chechens from Chechnya. It is the other way around, the c.358_360delGAG deletion was revealed in individuals from Chechnya only. The c.35delG mutation was found both in Ingushetia and Chechnya.
Table 4. Number (%, 95% CI) of heterozygous carriers of del(GJB2-D13S175) or known GJB2 mutations in apparently healthy populations.
| Mutation | Ingushes from Ingushetia, n=151 | Chechens from Ingushetia, n=90 | Chechens from Chechnya, n=147 |
|---|---|---|---|
| del(GJB2-D13S175) | 1 (0.7; 0.02–3.6) | 1 (1.1; 0.03–6.0) | 0 (0; 0–2.5) |
| c.35delG | 3 (2; 0.4–5.7) | 0 (0; 0–4.0) | 1 (0.7; 0.02–3.7) |
| c.358_360delGAG | 0 (0; 0–2.4) | 1 (1.1; 0.03–6.0) | 2 (1.4; 0.2–4.8) |
| c.313_326del14 | 0 (0; 0–2.4) | 0 (0; 0–4.0) | 0 (0; 0–2.5) |
| c.235delC | 0 (0; 0–2.4) | 0 (0; 0–4.0) | 0 (0; 0–2.5) |
Abbreviation: CI, confidence interval.
Age of del(GJB2-D13S175) estimation
Screening 3 STRs linked to GJB2 in eight patients carrying del(GJB2-D13S175) and 2 of these STRs in 90 healthy Ingushes, we identified a common founder haplotype for the del(GJB2-D13S175) allele and its decay derivatives (Table 5). The probable ‘founder haplotype’ is ‘15-22-11’ at D13S1316-D13S1275-D13S232. To obtain an estimate of the age of del(GJB2-D13S175), we used calculations for allele ‘22’ at D13S1275 and allele ‘11’ at D13S232, which in a statistically significant linkage disequilibrium (LD) with del(GJB2-D13S175). The average spreading time (age) of del(GJB2-D13S175) was estimated to be 100 (90% confidence interval: 40–300) generations, when calculations from Table 5 were generalized. Applying an average generation time of 30 years,28, 29, 30 we estimated del(GJB2-D13S175) to be ~3000 years old (90% confidence interval: 1000–9000).
Table 5. Haplotype analysis.
| Marker | D13S1316 | del(GJB2-D13S175) | D13S175 | D13S1275 | D13S232 |
|---|---|---|---|---|---|
| GRCh37, Mb | 20.68 | 20.76–20.86 | 20.85 | 22.95 | 23.80 |
| Marshfield, cM | 0 | 6.03 | 6.99 | 6.99 | |
| Genethon, cM | 0 | 7.4 | 8.8 | No data | |
| Chromosome | |||||
| Patient E | 15 | del | del | 22 | 11 |
| Patient H | 15 | del | del | 22 | 11 |
| Patient E | 2 | del | del | 22 | 11 |
| Patient D | 15 | del | del | 22 | 13 |
| Patient A | 15/14 | del | del | 22/24 | 11/19 |
| Patient F | 15 | del | del | 23 | 11/13 |
| Patient F | 15 | del | del | 25 | 13/11 |
| Patient H | 15 | del | del | 23 | 19 |
| Patient G | 15 | del | del | 20 | 19 |
| Patient B | 15/14 | del | del | 23/26 | 13 |
| Patient C | 15/14 | del | del | 21/19 | 13/20 |
| The healthy Ingush | 15/18 | del | del | 25/26 | 13/14 |
| Allele associated with del(GJB2-D13S175) | 15 | del | del | 22 | 11 |
| PD | 0.875 (0.917) | 0.455 (0.417) | 0.455 (0.417) | ||
| PN | 1/6=0.167a (1/10=0.1)a | 23/180=0.128 | 23/160=0.144 | ||
| P-valueb | 0.0353 (0.0007) | 0.015 (0.0202) | 0.0290 (0.0390) | ||
| δ; 90% CI | 0.850; 0.612–1.00 (0.908; 0.761–1.00) | 0.375; 0.042–0.708 (0.331; 0.062–0.601) | 0.363; 0.024–0.702 (0.319; 0.044–0.594) | ||
| gMarsh; 90% CI | No linkage map data | 102; 36–328 (114; 53–289) | 105; 37–386 (118; 54–325) | ||
| gGen; 90% CI | No linkage map data | 70; 25–224 (78; 36–197) | No linkage map data |
Abbreviation: CI, confidence interval.
The frequency obtained using the normal chromosomes from patients and their parents. δ, the degree of linkage disequilibrium by Bengtsson and Thomson,24 δ=(PD−PN)/(1−PN), where PD is the frequency of associated allele on del(GJB2-D13S175) carrying chromosomes and PN is the frequency of the same allele on chromosomes without del(GJB2-D13S175). g, the generation number by Risch et al.,22 obtained by use of θ values between D13S175 and D13S1275 or D13S232 from Marshfield map, gMarsh, and from Genethon map, gGen.
P-value for Yates corrected χ2.
At the top, the position of del(GJB2-D13S175) and four microsatellite markers studied. In the middle, haplotypes of del(GJB2-D13S175) carrying chromosomes. For patients A, B, C, F and the healthy Ingush, we were unable to phase mutation-carrying chromosomes into haplotypes by using other family members, therefore, two alleles are separated by slash. Below, the age of del(GJB2-D13S175) in the Ingushes is estimated. Within brackets, the calculations taking into account chromosomes of patients A, B, C, F and the healthy Ingush are present.
Discussion
Among the GJB2 variants reported previously, 252 missense substitutions, 54 small deletions, 26 nonsense substitutions, 18 small insertions, 7 small indels, 6 splice site substitutions, 5 large deletions, 3 substitutions in a regulatory sequence and 2 large insertions have been included in HGMD Professional 2016.2 as ‘disease-causing’ or ‘probable pathological’ mutations. Total of 100 and 39 null variants (nonsense, frameshift, canonical ±1 or 2 splice sites) have been registered as at January 2017 in HGMD and dbSNP, consequently. Among them, 27 variants were contained in dbSNP and in HGMD simultaneously, and were labeled ‘pathogenic’ or ‘likely pathogenic’ in ClinVar database. Remaining 12 null variants from dbSNP were absent in HGMD or ClinVar, but were found in the collection of ExAC. Thus, currently there is not any benign (or likely benign) null variant of GJB2. Among 252 missense mutations registered in HGMD, 158 substitutions have not been presented in dbSNP or other databases. At the same time, total of 161 GJB2 missense substitutions have been registered in dbSNP, which included 58 pathogenic (or likely pathogenic) and 11 benign (or likely benign) substitutions according to ClinVar. These data prove that missense variation in GJB2, as well as null variants, is a common cause of disease and there is little benign variation observed in the gene. Therefore, a novel missense variant in GJB2 can be considered supporting evidence for pathogenicity, and a novel null variant can be weighted as a very strong pathogenic criterion, according to the ACMG guidelines for classifying pathogenic variants.27 In this research, three unpublished pathogenic variants, namely, c.399G>A (p.Trp133*), c.402G>A (p.Trp134*), c.502_601del10 (p.Lys168Profs*5), and five novel likely pathogenic variants, c.94C>G (p.Arg32Gly), c.205T>C (p.Phe69Leu), c.245T>A (p.Ile82Asn), c.266T>C (p.Leu89Pro), c.532G>A (p.Val178Met), in the GJB2 gene were detected in Russian patients with moderately severe or severe hearing loss. Each of these variants were observed in a single family, an exception is c.266T>C, which was found in two unrelated families.
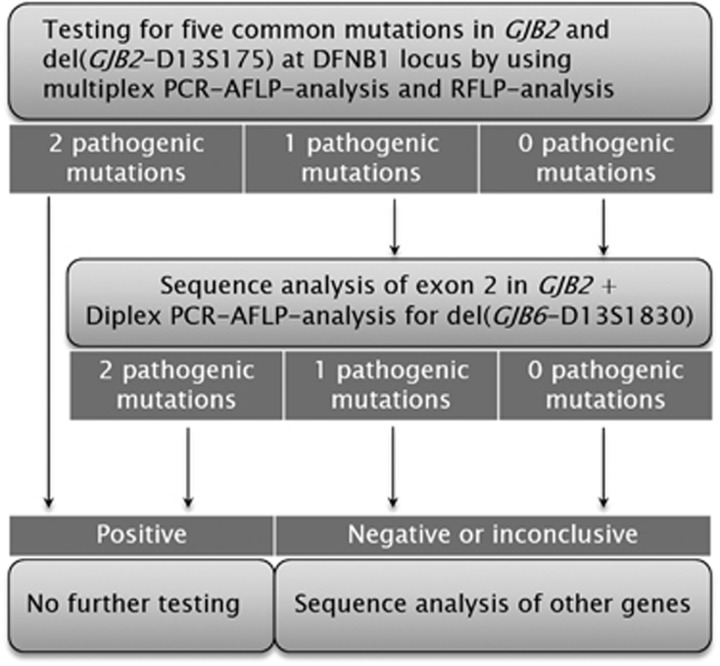
The most remarkable updating of the DFNB1 mutation spectrum is the novel del(GJB2-D13S175) deletion, recently described by us. A deletion del(GJB2-D13S175) as it was shown in this study is the most frequent among other large deletions at DFNB1 locus in Russia and is predominantly associated with profound deafness. The frequency of del(GJB2-D13S175) in our patients with hearing loss is twice higher than frequency of the well-known del(GJB6-D13S1830) deletion. So del(GJB2-D13S175) detection is important, as well as del(GJB6-D13S1830) detection, and has been added to our testing strategy for DFNB1 hearing loss as outlined in Figure 2. The del(GJB2-D13S175) identification used to detect a heterozygous carriage in normal hearing individuals is especially necessary since del(GJB2-D13S175) is missed by standard methods of GJB2 analysis, but it can be defined by using ALFP testing with deletion-specific breakpoint primers or copy-number analysis.
Figure 2.
The cost-optimized DFNB1 testing strategy. The common mutation test lay in two reactions, a multiplex PCR with primer pairs for six deletions (c.35delG, c.313_326del14, c.235delC, c.167delT, c.358_360delGAG and GJB2-D13S175) and RLFP analysis of c.−23+1G>A, followed by gel-based fragment analysis to differentiate wild-type and mutation alleles.
The del(GJB2-D13S175) mutation was originated from common founder in Ingush ethnic population. Among 241 normal hearing indigenous persons from Ingushetia, two heterozygous carriers for the deletion were observed (0.83% with 95% confidence interval 0.10%–2.97%), one can assume that there is unusually high carrier frequency of del(GJB2-D13S175) in Ingushetia. The similar frequency value of the c.35delG mutation was observed among normal hearing Ingushes. It is interesting that our cohort included a few patients with hearing loss from Ingushetia in addition to the patients with del(GJB2-D13S175). Among them, one out of two Ingush patients was homozygous for the c.35delG GJB2 variant, while the remaining one Ingush patient has no mutation in GJB2. Therefore we assume that among Ingushes, a frequency of the del(GJB2-D13S175) allele is close to the c.35delG frequency, both among normal hearing and deaf individuals.
The GJB2-D13S175 deletion was found in the population samples both among Ingushes and Chechens from Ingushetia, while this deletion was not observed among Chechens from Chechnya. The other c.358_360delGAG mutation tested here was observed among Chechens, both from Chechnya and Ingushetia, but was not revealed among Ingushes. Probably, spectrum and frequency of GJB2 mutations in Ingushes differ from that in Chechens from Chechnya, but Chechens from Ingushetia have mixed genetic material for this locus of both populations. This assumption agrees with findings, reported by Balanovsky et al.,31 which showed a high degree of genetic subdivision in the Caucasus populations resulting from genetic drift, probably due to isolation in the extremely mountainous landscape.
Study by Balanovsky et al.31 presents the most extensive survey of Y chromosomal variation in the Caucasus. Authors concluded that the Caucasus male lineages originated from a subset of the Near Eastern gene pool due to an Upper Paleolithic (or Neolithic) migration, followed by high levels of isolation, differentiation and genetic drift in situ. This process would result in the loss of some haplogroups and the increased frequency of others. Despite the fact that Ingushes and Chechens are nearest neighbors and had a shared ancestry for a long period, their languages and gene pools have been split ~2000 years ago, to what a linguistic history and age of a few specific haplotype clusters for Y chromosomal STRs in these populations testify.31 Our results demonstrate that the del(GJB2-D13S175) allele was spread in Ingushes after they separated from Chechens. In accordance with our average age estimates, the beginning of the del(GJB2-D13S175) spread among Ingushes occurred 1000 years before the linguistic division of Ingushes took place or the Ingush-specific Y chromosome haplotype clusters were originated.
It should be noted that at present over 25% of Ingushes live outside Russian Federation, mainly in Turkey, Syria, Jordan, Lebanon and Kazakhstan.32 The first mass migration of Ingushes and other North Caucasian ethnic groups to Ottoman Empire was happening during the second half of the nineteenth century after Caucasian War. During World War II, the whole of the Ingush and Chechen populations of the North Caucasus was temporarily deported to Kazakhstan and Central Asia, where they have partly remained and live to this day.33 Thus, the Ingush deletion can even quite possibly be found in countries mentioned above. It is possible also that the deletion has been already present among Ingush ancestors, for example, among Neolithic/Bronze age migrants from the Near East, before the Ingush people were isolated. Therefore, to establish whether the deletion is unique for the Ingushes, additional researches are necessary. It is important both for clinical genetics and for the Caucasus history study.
Acknowledgments
We are grateful to the patients and their parents for their participation. EAB thanks Prof Georgy A Tavartkiladze for critical reading of the manuscript and Alyona L Chukhrova, Svetlana I Braslavskaya, Nina Ryadninskaya, Gulnara Bayazutdinova for their help in the sequencing analysis.
Footnotes
Supplementary Information accompanies the paper on Journal of Human Genetics website (http://www.nature.com/jhg)
The authors declare no conflict of interest.
Supplementary Material
References
- Tsukada, K., Nishio, S. Y., Hattori, M. & Usami, S. Ethnic-specific spectrum of GJB2 and SLC26A4 mutations: their origin and a literature review. Ann. Otol. Rhinol. Laryngol. 124 (Suppl 1), 61S–76S (2015). [DOI] [PubMed] [Google Scholar]
- del Castillo, I., Villamar, M., Moreno-Pelayo, M. A., del Castillo, F. J., Alvarez, A., Telleria, D. et al. A deletion involving the connexin 30 gene in nonsyndromic hearing impairment. N. Engl. J. Med. 346, 243–249 (2002). [DOI] [PubMed] [Google Scholar]
- Lerer, I., Sagi, M., Ben-Neriah, Z., Wang, T., Levi, H. & Abeliovich, D. A deletion mutation in GJB6 cooperating with a GJB2 mutation in trans in non-syndromic deafness: a novel founder mutation in Ashkenazi Jews. Hum. Mutat. 18, 460 (2001). [DOI] [PubMed] [Google Scholar]
- Pallares-Ruiz, N., Blanchet, P., Mondain, M., Claustres, M. & Roux, A. F. A large deletion including most of GJB6 in recessive non syndromic deafness: a digenic effect? Eur. J. Hum. Genet. 10, 72–76 (2002). [DOI] [PubMed] [Google Scholar]
- Del Castillo, I., Moreno-Pelayo, M. A., Del Castillo, F. J., Brownstein, Z., Marlin, S., Adina, Q. et al. Prevalence and evolutionary origins of the del(GJB6-D13S1830) mutation in the DFNB1 locus in hearing-impaired subjects: a multicenter study. Am. J. Hum. Genet. 73, 1452–1458 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Castillo, F. J., Rodriguez-Ballesteros, M., Alvarez, A., Hutchin, T., Leonardi, E., de Oliveira, C. A. et al. A novel deletion involving the connexin-30 gene, del(GJB6-d13s1854), found in trans with mutations in the GJB2 gene (connexin-26) in subjects with DFNB1 non-syndromic hearing impairment. J. Med. Genet. 42, 588–594 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann, D., Le Marechal, C., Jonard, L., Thierry, P., Czajka, C., Couderc, R. et al. A new large deletion in the DFNB1 locus causes nonsyndromic hearing loss. Eur. J. Med. Genet. 52, 195–200 (2009). [DOI] [PubMed] [Google Scholar]
- Tayoun, A. N., Mason-Suares, H., Frisella, A. L., Bowser, M., Duffy, E., Mahanta, L. et al. Targeted droplet-digital PCR as a tool for novel deletion discovery at the DFNB1 locus. Hum. Mutat. 37, 119–126 (2016). [DOI] [PubMed] [Google Scholar]
- Wilch, E., Azaiez, H., Fisher, R. A., Elfenbein, J., Murgia, A., Birkenhager, R. et al. A novel DFNB1 deletion allele supports the existence of a distant cis-regulatory region that controls GJB2 and GJB6 expression. Clin. Genet. 78, 267–274 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliznetz, E. A., Makienko, O. N., Okuneva, E. G., Markova, T. G. & Polyakov, A. V. New recurrent large deletion, encompassing both GJB2 and GB6 genes, results in isolated sensorineural hearing impairment with autosomal recessive mode of inheritance. Russ. J. Genet. 50, 415–420 (2014). [PubMed] [Google Scholar]
- Rodriguez-Paris, J. & Schrijver, I. The digenic hypothesis unraveled: the GJB6 del(GJB6-D13S1830) mutation causes allele-specific loss of GJB2 expression in cis. Biochem. Biophys. Res. Commun. 389, 354–359 (2009). [DOI] [PubMed] [Google Scholar]
- Rodriguez-Paris, J., Tamayo, M. L., Gelvez, N. & Schrijver, I. Allele-specific impairment of GJB2 expression by GJB6 deletion del(GJB6-D13S1854). PLoS ONE 6, e21665 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilch, E., Zhu, M., Burkhart, K. B., Regier, M., Elfenbein, J. L., Fisher, R. A. et al. Expression of GJB2 and GJB6 is reduced in a novel DFNB1 allele. Am. J. Hum. Genet. 79, 174–179 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Common, J. E., Bitner-Glindzicz, M., O'Toole, E. A., Barnes, M. R., Jenkins, L., Forge, A. et al. Specific loss of connexin 26 expression in ductal sweat gland epithelium associated with the deletion mutation del(GJB6-D13S1830). Clin. Exp. Dermatol. 30, 688–693 (2005). [DOI] [PubMed] [Google Scholar]
- Putcha, G. V., Bejjani, B. A., Bleoo, S., Booker, J. K., Carey, J. C., Carson, N. et al. A multicenter study of the frequency and distribution of GJB2 and GJB6 mutations in a large North American cohort. Genet. Med. 9, 413–426 (2007). [DOI] [PubMed] [Google Scholar]
- Pandya, A., Arnos, K. S., Xia, X. J., Welch, K. O., Blanton, S. H., Friedman, T. B. et al. Frequency and distribution of GJB2 (connexin 26) and GJB6 (connexin 30) mutations in a large North American repository of deaf probands. Genet. Med. 5, 295–303 (2003). [DOI] [PubMed] [Google Scholar]
- Dalamon, V., Beheran, A., Diamante, F., Pallares, N., Diamante, V. & Elgoyhen, A. B. Prevalence of GJB2 mutations and the del(GJB6-D13S1830) in Argentinean non-syndromic deaf patients. Hear. Res. 207, 43–49 (2005). [DOI] [PubMed] [Google Scholar]
- Markova, T. G., Nekrasovan, V. M., Shagina, I. A. & Poliakov, A. A. Genetic screening among children with congenital and early childhood hearing loss. Vestn. Otorinolaringol. 4, 9–14 (2006). [PubMed] [Google Scholar]
- Bliznetz, E. A., Galkina, V. A., Matyushchenko, G. N., Kisina, A. G., Markova, T. G. & Polyakov, A. V. Changes in the connexin 26 gene (GJB2 in Russian patients with hearing loss: results of long-term molecular diagnostics of hereditary nonsyndromic hearing loss. Russ. J. Genet. 48, 101–112 (2012). [PubMed] [Google Scholar]
- Zinchenko, R. A., Osetrova, A. A. & Sharonova, E. I. Hereditary deafness in Kirov oblast: estimation of the incidence rate and DNA diagnosis in children. Russ. J. Genet. 48, 455–462 (2012). [PubMed] [Google Scholar]
- Lalaiants, M. R., Markova, T. G., Bakhshinian, V. V., Bliznets, E. A., Poliakov, A. V. & Tavartikiladze, G. A. The audiological phenotype and the prevalence of GJB2-related sensorineural loss of hearing in the infants suffering acoustic disturbances. Vestn. Otorinolaringol. 2, 37–43 (2014). [PubMed] [Google Scholar]
- Balanovska, E. V., Zhabagin, M. K., Agdzhoyan, A. T., Chukhryaeva, M. I., Markina, N. V., Balaganskaya, O. A. et al. Population biobanks: organizational models and prospects of application in gene geography and personalized medicine. Russ. J. Genet. 52, 1227–1243 (2016). [Google Scholar]
- Risch, N., de Leon, D., Ozelius, L., Kramer, P., Almasy, L., Singer, B. et al. Genetic analysis of idiopathic torsion dystonia in Ashkenazi Jews and their recent descent from a small founder population. Nat. Genet. 9, 152–159 (1995). [DOI] [PubMed] [Google Scholar]
- Bliznetz, E. A., Tverskaya, S. M., Zinchenko, R. A., Abrukova, A. V., Savaskina, E. N., Nikulin, M. V. et al. Genetic analysis of autosomal recessive osteopetrosis in Chuvashiya: the unique splice site mutation in TCIRG1 gene spread by the founder effect. Eur. J. Hum. Genet. 17, 664–672 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson, B. O. & Thomson, G. Measuring the strength of associations between HLA antigens and diseases. Tissue Antigens 18, 356–363 (1981). [DOI] [PubMed] [Google Scholar]
- Diaz, G. A., Gelb, B. D., Risch, N., Nygaard, T. G., Frisch, A., Cohen, I. J. et al. Gaucher disease: the origins of the Ashkenazi Jewish N370S and 84GG acid beta-glucosidase mutations. Am. J. Hum. Genet. 66, 1821–1832 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards, S., Aziz, N., Bale, S., Bick, D., Das, S., Gastier-Foster, J. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17, 405–424 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgason, A., Hrafnkelsson, B., Gulcher, J. R., Ward, R. & Stefansson, K. A populationwide coalescent analysis of Icelandic matrilineal and patrilineal genealogies: evidence for a faster evolutionary rate of mtDNA lineages than Y chromosomes. Am. J. Hum. Genet. 72, 1370–1388 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocheshkhova, E. A., Balanovska, E. V., Seregin, Y. A., Golubtsov, V. L. & Balanovsky, O. P. Temporal dynamics of gene pool reconstructed from genealogical and surname data. Med. Genet. 7, 25–29 (2008). [Google Scholar]
- Tremblay, M. & Vezina, H. New estimates of intergenerational time intervals for the calculation of age and origins of mutations. Am. J. Hum. Genet. 66, 651–658 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balanovsky, O., Dibirova, K., Dybo, A., Mudrak, O., Frolova, S., Pocheshkhova, E. et al. Parallel evolution of genes and languages in the Caucasus region. Mol. Biol. Evol. 28, 2905–2920 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolgieva, M. B. A modern settlement of Ingushes. Vopr. Istor. 10, 86–87 (2013). [Google Scholar]
- Kodzoev, N. D. (ed) Ingushetia Hystory (Tetragraf Ltd., Magas-Naltchik, 2011).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.