Figure 2. Overall Survival.
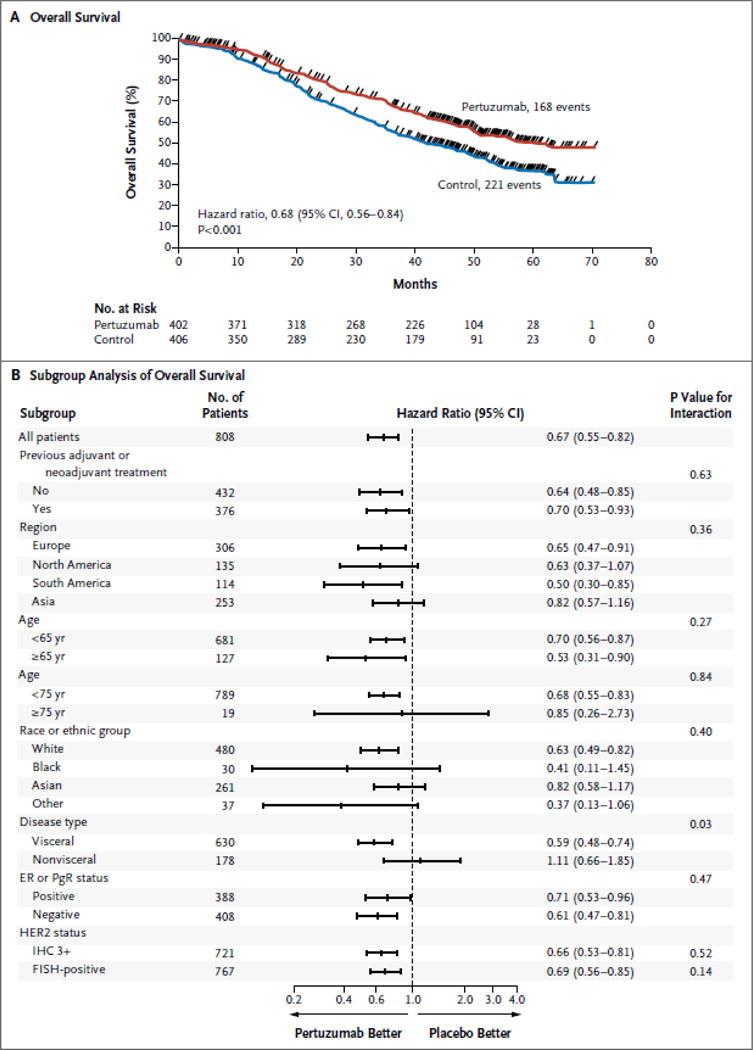
Panel A shows Kaplan–Meier estimates of overall survival in the intention-to-treat population, stratified according to adjuvant or neoadjuvant therapy and geographic region. The median overall survival among patients receiving pertuzumab, trastuzumab, and docetaxel (pertuzumab group) was 56.5 months, 15.7 months longer than survival among patients receiving placebo, trastuzumab, and docetaxel (control group). The tick marks indicate censoring events. Panel B shows hazard ratios and 95% confidence intervals for overall survival in all prespecified subgroups according to baseline characteristics, without stratification. Race or ethnic group was determined by the investigator. “Other” includes American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, and other populations. A grade of 3+ on immunohistochemical (IHC) analysis indicates positivity for human epidermal growth factor receptor 2 (HER2). For the assessment of HER2 status, prespecified subgroup analyses were restricted to patients whose tumors had an IHC score of 3+ or fluorescence in situ hybridization (FISH) positive status, since these categories accounted for approximately 90% of patients. CI denotes confidence interval, ER estrogen receptor, and PgR progesterone receptor.
