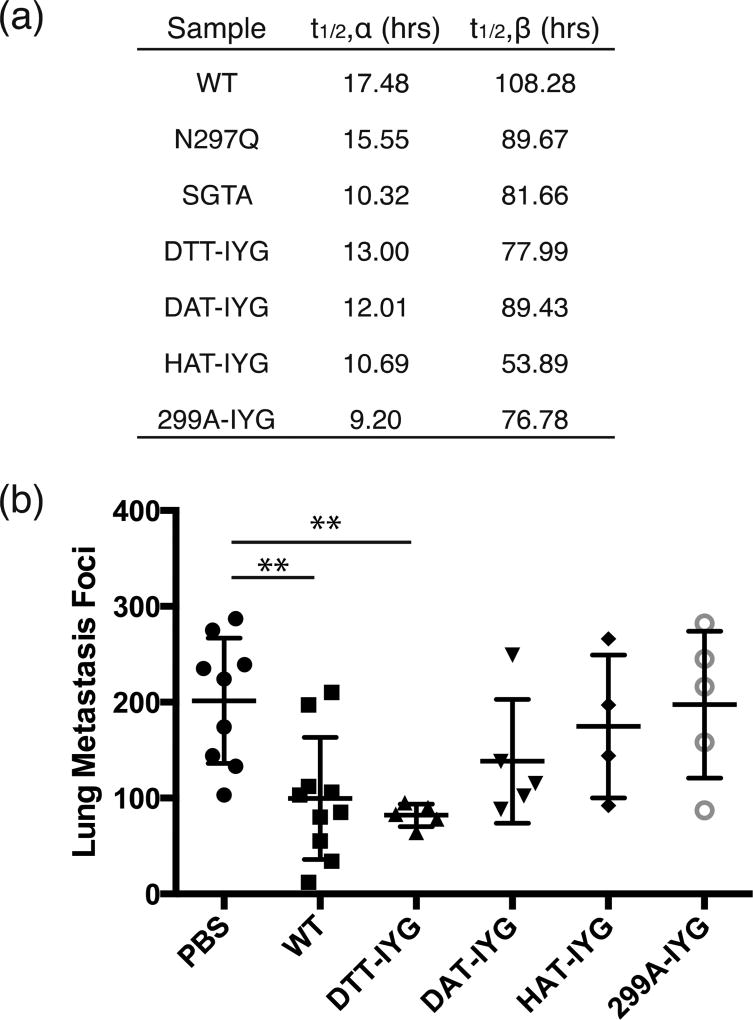
Figure 4.
In vivo pharmacokinetic characterization and lung metastasis reduction with aglycosylated variants. (a) Table of alpha half-lives and beta half-lives of each aglycosylated antibody based on pharmacokinetic curves. Readings are fit to: C(t) = Ae−αt + Be−βt. Each study was done in triplicate. (b) Aglycosylated antibodies were used to treat B16F10 lung metastases. Wild-type (WT) glycosylated IgG1 serves as the positive control and PBS serves as the negative vehicle control. ** p-val < 0.01 as compared to negative control of PBS treatment. Other treatments were not significantly different from PBS. DTT-IYG was not significantly different from WT treatment. Five to 10 mice were used per group.