Figure 1.
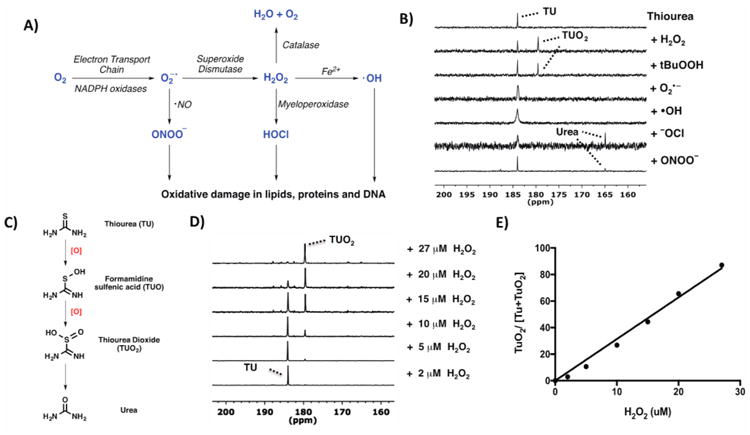
(A) Different kinds of ROS generated via multiple enzymatic steps. (B) Response of thiourea (TU) to various ROS at thermal equilibrium. All ROS (H2O2, tBuOOH, NaONOO, and NaOCl) were added to TU in D2O (except for KO2-treated sample in DMSO) and the spectra recorded with 13C NMR at 125 mHz. Reaction with H2O2 and tBuOOH yielded thiourea dioxide (TUO2) as the oxidized product. (C) TU was oxidized to TUO2 before getting hydrolyzed to urea. (D) 13C NMR spectra of 10 μM 13C-TU after 10 min reaction with various concentrations of H2O2 (2–27 μM). (E) Linear correlation of the ratio of integrated peak intensities of 13C-TUO2 to the total signal intensities (13C-TU + 13C-TUO2) against H2O2 concentration, R2 = 0.994.
