Abstract
IMPORTANCE
Hematoma expansion is a potentially modifiable predictor of poor outcome following an acute intracerebral hemorrhage (ICH). The ability to identify patients with ICH who are likeliest to experience hematoma expansion and therefore likeliest to benefit from expansion-targeted treatments remains an unmet need. Hypodensities within an ICH detected by noncontrast computed tomography (NCCT) have been suggested as a predictor of hematoma expansion.
OBJECTIVE
To determine whether hypodense regions, irrespective of their specific patterns, are associated with hematoma expansion in patients with ICH.
DESIGN, SETTING, AND PARTICIPANTS
We analyzed a large cohort of 784 patients with ICH (the development cohort; 55.6% female), examined NCCT findings for any hypodensity, and replicated our findings on a different cohort of patients (the replication cohort; 52.7% female). Baseline and follow-up NCCT data from consecutive patients with ICH presenting to a tertiary care hospital between 1994 and 2015 were retrospectively analyzed. Data analyses were performed between December 2015 and January 2016.
MAIN OUTCOMES AND MEASURES
Hypodensities were analyzed by 2 independent blinded raters. The association between hypodensities and hematoma expansion (>6 cm3 or 33% of baseline volume) was determined by multivariable logistic regression after controlling for other variables associated with hematoma expansion in univariate analyses with P ≤ .10.
RESULTS
A total of 1029 patients were included in the analysis. In the development and replication cohorts, 222 of 784 patients (28.3%) and 99 of 245 patients (40.4%; 321 of 1029 patients [31.2%]), respectively, had NCCT scans that demonstrated hypodensities at baseline (κ = 0.87 for interrater reliability). In univariate analyses, hypodensities were associated with hematoma expansion (86 of 163 patients with hematoma expansion had hypodensities [52.8%], whereas 136 of 621 patients without hematoma expansion had hypodensities [21.9%]; P < .001). The association between hypodensities and hematoma expansion remained significant (odds ratio, 3.42 [95%CI, 2.21–5.31]; P < .001) in a multivariable model; other independent predictors of hematoma expansion were a CT angiography spot sign, a shorter time to CT, warfarin use, and older age. The independent predictive value of hypodensities was again demonstrated in the replication cohort (odds ratio, 4.37 [95%CI, 2.05–9.62]; P < .001).
CONCLUSION AND RELEVANCE
Hypodensities within an acute ICH detected on an NCCT scan may predict hematoma expansion, independent of other clinical and imaging predictors. This novel marker may help clarify the mechanism of hematoma expansion and serve as a useful addition to clinical algorithms for determining the risk of and treatment stratification for hematoma expansion.
Spontaneous intracerebral hemorrhage (ICH) is the most deadly type of stroke,1 with an up to 40% mortality rate at 30 days and only one-fifth of the survivors being independent after 6 months.2 Although many determinants of ICH outcome, such as location and baseline volume, are non-modifiable at presentation,3,4 clinically significant hematoma expansion, occurring in more than a third of patients with ICH,5 is an independent prognostic factor6 and a potential therapeutic target.7–10 However, treatments aimed at limiting hemorrhage expansion have yet to yield improved outcomes in clinical trials.7,11–13 Refining our ability to identify those patients who are most likely to benefit from expansion-targeted treatments could have important implications for clinical practice and future trials.11
Recent studies have synthesized clinically applicable ICH expansion prediction scores, using known predictors such as baseline volume, time to diagnosis, anticoagulation with warfarin, intraventricular extension,14,15 and the computed tomography angiography (CTA) spot sign.16 Although the CTA spot sign is one of the strongest predictors of expansion,17,18 its assessment requires a CTA, which is not routinely performed in the acute phase in many centers.19
Previous studies have examined whether the findings of noncontract CT (NCCT), an almost universally available test in the acute setting, can independently predict expansion. Some have found that hypodensities within the hematoma and ICH heterogeneity, sometimes referred to as the “swirl sign,” can serve as such predictors in both spontaneous and traumatic ICH.20–25 Other studies have reported that an irregular shape or the heterogeneity of a hematoma (ie, the presence of a “blend sign”) can be helpful.21,26–29
Unfortunately, these different findings have different definitions, with variable interrater reliability, and often do not compare their findings with other predictors (such as warfarin status or spot sign). It is not clear how often they overlap, and it may be that they are capturing different aspects of the same ICH pathophysiology cascade.
We hypothesized that hypodense regions within the hematoma, irrespective of their specific patterns, would predict hematoma expansion. We analyzed a large cohort of patients with ICH, examined NCCT findings for any hypodensity within the hematoma, and compared the predictive ability of this finding with previously described NCCT predictors, as well as the CTA spot sign.
Methods
Study Population
Consecutive patients with primary ICH who were admitted to a single academic tertiary care medical center were included in an ongoing prospective cohort study, as previously described.17,30 Patients were included in this retrospective analysis if they underwent a baseline and a follow-up CT within 48 hours of symptom onset, and if CT scans of adequate quality were available. Patients with primary intraventricular hemorrhage (IVH), a baseline ICH volume of less than 1 cm3, or patients who had been enrolled in randomized clinical trials of aggressive blood pressure–lowering treatment, as well as patients who underwent surgery prior to follow-up CT, were excluded. Consecutive patients admitted between 1994 and 2012 were assigned to the development cohort, and those admitted between 2013 and 2015 were assigned to the replication cohort.
Clinical Data
Recruitment and data collection have been previously described.30 In brief, clinical data (including age, sex, medical history, and previous medication use) were all obtained through interviews with the patients (or their surrogates) by trained study staff. Prospectively recorded admission variables comprised the Glasgow Coma Scale score, systolic and diastolic blood pressure, and time from symptom onset to baseline and follow-up CT. All patients were treated according to a standard institutional protocol during the recruitment period (current version available online at https://www2.massgeneral.org/stopstroke/treatmentProtocols.aspx). The institutional review board of Massachusetts General Hospital approved this study, and the patients provided written informed consent.
Imaging Data and Analysis
Neuroradiologists or neurologists (blinded to clinical and outcome data) ascertained hemorrhage locations. For the development and replication cohorts, the ICH volumes obtained from the baseline and follow-up CT scans were prospectively calculated by study staff blinded to the data according to standard protocols using available software (Alice; PAREXEL International Corporation31 and Analyze 10.0; Mayo Clinic32). Spot sign reading was performed as previously described.30 Significant hematoma expansion was defined as an increase in volume between baseline and follow-up CT exceeding 6 cm3 or 33% of the baseline volume.33
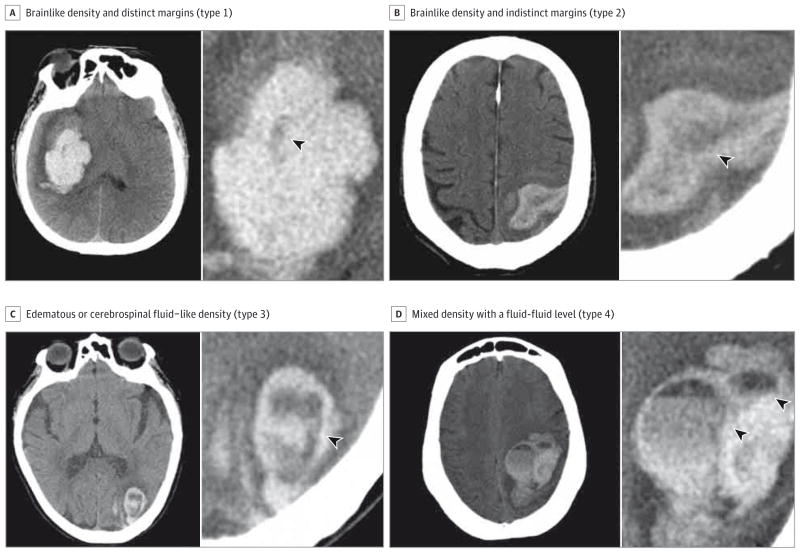
A first joint reading of 100 patients’ baseline NCCT scans led to the description of 4 types of hypodensities, empirically defined based on the distinctness of their margins and their relative density: type 1 hypodensity has a brain like density and distinct margins, type 2 has a brain like density and indistinct margins, type 3 has an edematous or cerebrospinal fluid–like density, and type 4 has a mixed density with a fluid-fluid level (Figure 1). Hypodensities connected to the outer surface of the hematoma were excluded to avoid partial volume errors (eFigure 1 in the Supplement). For the purposes of the overall analysis, the presence of at least 1 of any of these types of hypodensities was considered positive for “hypodensity.”
Figure 1. Illustrative Examples of 4 Types of Hypodensities.
Axial sections of noncontrast computed tomographic scans revealing hypodensities of various aspects inside acute intracerebral hemorrhages (arrowheads).
The baseline NCCT scans of eligible patients in both cohorts were reviewed by 2 trained raters, a neuroradiologist (the first reader, G.B.) and a stroke neurologist (A.M.), who were blinded to other data and who assessed the presence of hypodensities and their categorization, as well as previously published NCCT predictors of expansion: irregularity and homogeneity of hematoma according to a 5-point scale,21 presence of a fluid level (defined as the presence of distinct areas within the hematoma, separated by a linear interface),27 and presence of a blend sign.28 The NCCT scans were assessed using a fixed window of 110 Hounsfield units and a level of 50 Hounsfield units.
Statistical Analysis
Continuous variables were summarized using mean (SD) values or median (interquartile range [IQR]) values as appropriate, and discrete variables were summarized using counts (percentages). The χ2 test, the Fisher exact test, the t test, and the Mann-Whitney test were used as appropriate for the univariate analysis, with P < .05 as the threshold for statistical significance. Multivariable logistic regression models were used to determine factors that were independently associated with significant hematoma expansion. Variables associated with the outcome in univariate analysis (P ≤ .10) were entered into the nominal logistic model, adjusting for age and sex, and then backward elimination was used to remove non significant variables (P > .05). Agreement statistics (interrater and intrarater reliability) on categorical variables were performed using the Cohen κ interagreement test.34 All analyses were performed using JMP Pro 12 software (SAS Institute Inc). This report was prepared according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.35
Results
Study Population
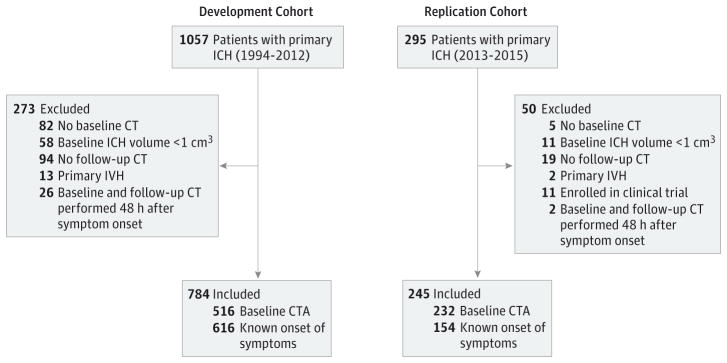
After application of the eligibility criteria, 784 of 1057 patients with primary ICH in the development cohort and 245 of 295 patients with primary ICH in the replication cohort (for a total of 1029 patients) were included in the analysis (Figure 2). The baseline clinical characteristics were comparable between the development and replication cohorts, although there were differences in time from symptom onset to baseline CT (with a median time of 4.9 hours [IQR, 2.5–8.1 hours] for patients in the development cohort vs 3.2 hours [IQR, 1.1–4.6 hours] for patients in the replication cohort; P < .001), presence of spot sign (94 of 516 patients in the development cohort [18.2%] vs 74 of 236 patients in the replication cohort [31.4%]; P < .001), and presence of hypodensity (222 of 784 patients in the development cohort [28.3%] vs 99 of 245 patients in the replication cohort [40.4%]; P < .001). Patients with hypodensities had lower GCS scores at admission, used warfarin more frequently, had shorter times to CT, larger baseline ICH volumes, and more frequent spot signs (all P < .05) (Table 1).
Figure 2.
Flowchart of Study Patients
Table 1.
Clinical and Radiological Characteristics of Patients in the Development and Replication Cohorts
| Characteristic | Patients, No. (%) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Development Cohort (n = 784) | Replication Cohort (n = 245) | |||||
|
|
|
|||||
| Hypodensities | No Hypodensities | P Value | Hypodensities | No Hypodensities | P Value | |
| Total No. (%) | 222 (28.3) | 562 (71.7) | 99 (40.4) | 146 (59.6) | ||
|
| ||||||
| Age, mean (SD), y | 70.1 (13.3) | 72.4 (12.6) | .98 | 71.8 (11.4) | 72.4 (12.5) | .71 |
|
| ||||||
| Female sex | 131 (59.0) | 305 (54.3) | .23 | 55 (55.6) | 74 (50.7) | .52 |
|
| ||||||
| Diabetes | 48 (21.6) | 124 (22.1) | .92 | 20 (20.2) | 27 (18.5) | .87 |
|
| ||||||
| Hypertension | 45 (20.3) | 112 (19.9) | .92 | 27 (27.3) | 27 (18.5) | .16 |
|
| ||||||
| Atrial fibrillation | 55 (24.8) | 104 (18.5) | .06 | 26 (26.3) | 16 (11.0) | .003 |
|
| ||||||
| Hyperlipidemia | 83 (37.4) | 206 (36.7) | .80 | 45 (45.5) | 64 (43.8) | .90 |
|
| ||||||
| Antiplatelet therapy | 78 (35.1) | 264 (47.0) | .004 | 29 (29.3) | 41 (28.1) | .27 |
|
| ||||||
| Warfarin use | 71 (32.0) | 92 (16.4) | <.001 | 24 (24.2) | 18 (12.3) | .03 |
|
| ||||||
| INR, median (IQR) | 1.1 (1.0–2.4) | 1.1 (1.0–1.2) | <.001 | 1.1 (1.0–1.3) | 1 (1–1.1) | <.001 |
|
| ||||||
| GCS score, median (IQR) | 12 (7–15) | 14 (10–15) | <.001 | 11 (6–15) | 15 (12.3–15) | <.001 |
|
| ||||||
| SBP, mean (SD), mm Hg | 179.7 (35.7) | 178.8 (34.6) | .37 | 173.9 (33.2) | 172.2 (34.0) | .70 |
|
| ||||||
| Time from symptom onset to baseline CT,a median (IQR), h |
3.4 (1.6–5.0) | 5.5 (3.1–8.0) | <.001 | 1.2 (0.9–2.5) | 3.0 (1.9–6.1) | <.001 |
|
| ||||||
| Baseline ICH volume, mean (SD), cm3 | 44.1 (32.6) | 19.9 (23.0) | <.001 | 46.2 (33.7) | 19.2 (21.7) | <.001 |
|
| ||||||
| Baseline IVH volume, mean (SD), cm3 | 8.8 (18) | 4.9 (12.4) | .002 | 8.1 (25.9) | 5.8 (14.1) | <.001 |
|
| ||||||
| Presence of spot sign,b No./Total No. (%) | 58/136 (42.6) | 36/380 (9.5) | <.001 | 44/94 (46.8) | 30/142 (21.1) | <.001 |
|
| ||||||
| Hematoma expansion | 86 (38.7) | 62 (11.0) | <.001 | 43 (43.4) | 17 (11.6) | <.001 |
Abbreviations: GCS, Glasgow Coma Scale; ICH, intracerebral hemorrhage; INR, international normalized ratio; IVH, intraventricular hemorrhage; SBP, systolic blood pressure.
Data available for 616 patients in the development and 154 patients in the replication cohort.
Data available for 516 patients in the development and 236 patients in the replication cohort.
Computed Tomography
Of 784 patients in the development cohort, 222 (28.3%) had hypodensities. Excellent interrater agreement (κ = 0.87 [95% CI, 0.77–0.97]) and intrarater agreement (κ = 0.92 [95% CI, 0.79–0.98]) were obtained for the presence of hypodensities. Patients with hypodensities had larger ICH and IVH volumes, more frequently had spot signs, and more frequently had hematoma expansion on univariate analysis (all P < .05) (Table 1). Initial analysis of the development cohort showed no significant differences in the predictive values of the individual hypodensity types for expansion, and therefore the 4 types of hypodensities were lumped together for multivariable models and replication testing.
Predictors of Hematoma Expansion
In univariate analysis, warfarin use, CTA spot sign, larger baseline ICH and IVH volumes, shorter time to initial CT (≤6 hours), and presence of hypodensities were associated with hematoma expansion in both cohorts (Table 2) and were entered into a multivariable logistic regression model. All but baseline ICH and IVH volumes remained significant in multivariable analysis (Table 3).
Table 2.
Univariate Analysis of Hematoma Expansion
| Characteristic | Patients, No. (%)
|
|||||
|---|---|---|---|---|---|---|
| Development Cohort (n = 784)
|
Replication Cohort (n = 245)
|
|||||
| Hematoma Expansion | No Expansion | P Value | Hematoma Expansion | No Expansion | P Value | |
| Total No. (%) | 163 (20.8) | 621 (79.2) | 61 (24.9) | 184 (75.1) | ||
|
| ||||||
| Age, mean (SD), y | 73.4 (11.2) | 71.3 (13.2) | .06 | 73.4 (11.2) | 71.3 (13.2) | .16 |
|
| ||||||
| Female sex | 54 (33.1) | 294 (47.3) | .04 | 27 (44.3) | 88 (47.8) | .70 |
|
| ||||||
| Warfarin use | 51 (31.3) | 112 (18.0) | <.001 | 19 (31.1) | 20 (10.9) | <.001 |
|
| ||||||
| SBP, mean (SD), mm Hg | 173.5 (35.5) | 180.3 (34.6) | .12 | 176.8 (35.4) | 171.7 (33.2) | .16 |
|
| ||||||
| GCS score, mean (SD) | 11 (4.2) | 11 (4) | .14 | 9.6 (5) | 11 (3.9) | .02 |
|
| ||||||
| Time from symptom onset to baseline CT | ||||||
|
| ||||||
| ≤6 h (Reference) | 96 (58.9) | 297 (47.8) | 44 (72.1) | 85 (46.2) | ||
|
| ||||||
| >6 h | 27 (16.6) | 196 (31.6) | <.001 | 3 (4.9) | 26 (14.1) | <.001 |
|
| ||||||
| Unknown | 25 (15.3) | 143 (23.0) | 13 (21.3) | 72 (39.1) | ||
|
| ||||||
| Baseline ICH volume, mean (SD), cm3 | 34.6 (31.3) | 25 (27.2) | <.001 | 40.1 (33.4) | 26.7 (28.5) | .003 |
|
| ||||||
| Baseline IVH volume, mean (SD), cm3 | 8.6 (20.6) | 5.4 (12.3) | <.001 | 10.7 (33.2) | 5.5 (12.8) | .12 |
|
| ||||||
| Presence of spot sign,a No./Total No. (%) | 40/91 (44.0) | 54/425 (12.7) | <.001 | 34/57 (59.6) | 39/177 (22.0) | <.001 |
|
| ||||||
| Hypodensities | 86 (52.8) | 136 (21.9) | <.001 | 43 (70.5) | 55 (29.9) | <.001 |
|
| ||||||
| Type 1 | 41 (25.2) | 57 (9.2) | <.001 | 21 (34.4) | 31 (16.8) | .01 |
|
| ||||||
| Type 2 | 61 (37.4) | 70 (11.3) | <.001 | 26 (42.6) | 25 (13.6) | <.001 |
|
| ||||||
| Type 3 | 25 (15.3) | 52 (8.4) | <.001 | 14 (23.0) | 14 (7.6) | .002 |
|
| ||||||
| Type 4 | 12 (7.4) | 10 (1.6) | .003 | 7 (11.5) | 3 (1.6) | .003 |
|
| ||||||
| Swirl signb | 52 (31.9) | 89 (14.3) | <.001 | 27 (44.3) | 38 (20.7) | <.001 |
|
| ||||||
| Blend signc | 27 (16.6) | 84 (13.5) | .12 | 5 (8.2) | 25 (13.6) | .11 |
|
| ||||||
| Heterogeneousd | 62 (38.0) | 110 (17.7) | <.001 | 42 (68.9) | 67 (36.4) | <.001 |
|
| ||||||
| Irregulard | 93 (57.1) | 275 (44.3) | <.001 | 45 (73.8) | 88 (47.8) | <.001 |
|
| ||||||
| Fluid level | 7 (4.3) | 29 (4.7) | >.99 | 7 (11.5) | 53 (28.8) | .002 |
Abbreviations: CT, computed tomography; GCS, Glasgow Coma Scale; ICH, intracerebral hemorrhage; IVH, intraventricular hemorrhage; SBP, systolic blood pressure.
Data available for 154 patients in the development and 232 patients in the replication cohort.
According to Selariu et al.22
According to Li et al.28
According to Barras et al21 (score for heterogeneity/irregularity ≥3).
Table 3.
Multivariable Analysis of Hematoma Expansiona
| Variable | Development Cohort (n = 784)
|
Replication Cohort (n = 245)
|
||
|---|---|---|---|---|
| AOR (95% CI) | P Value | AOR (95% CI) | P Value | |
| Hypodensities | 3.42 (2.21–5.31) | <.001 | 4.37 (2.05–9.62) | <.001 |
|
| ||||
| Presence vs absence of spot sign | 2.72 (1.56–4.75) | <.001 | 3.34 (1.62–7.00) | <.001 |
|
| ||||
| Warfarin use | 1.99 (1.27–3.11) | <.001 | 3.43 (1.47–8.20) | <.001 |
|
| ||||
| <6 vs ≥6 h From symptom onset to baseline CT | 1.82 (1.11–3.07) | .03 | 4.64 (1.23–24.02) | .01 |
|
| ||||
| Baseline ICH volumeb | 1.17 (0.27–4.69) | .83 | 0.97 (0.14–6.58) | .98 |
|
| ||||
| Baseline IVH volumeb | 2.12 (0.36–11.6) | .40 | 1.84 (0.20–13.19) | .57 |
Abbreviations: AOR, adjusted odds ratio; CT, computed tomography; ICH, intracerebral hemorrhage; IVH, intraventricular hemorrhage.
Model is adjusted for age and sex.
Per change in regressor over entire range.
The presence of hypodensity was an independent predictor of expansion (adjusted OR, 3.42 [95% CI, 2.21–5.31]; P < .001), with the following diagnostic performances for expansion: a sensitivity of 0.62, a specificity of 0.77, and positive and negative predictive values of 0.4 and 0.89, respectively. Further adjusting for antiplatelet therapy in the multivariable model (associated with a lower prevalence of hypodensities in the development cohort; P = .04) did not change the predictive value of hypodensities for expansion (data not shown). The presence of hypodensities was independently associated with a shorter time to CT (P = .002), after adjustment for hemorrhage volume, spot sign, and/or IVH.
Other NCCT Markers of Expansion
Other NCCT markers previously reported to be predictors of expansion were evaluated in our cohorts (eTable in the Supplement). These markers were then added to the multivariable model. Among them, an irregular shape21,27 (adjusted OR, 1.72 [95%CI, 1.07–2.76]; P = .02) and the presence of a blend sign28 (adjusted OR, 2.04 [95% CI, 1.16–3.54]; P = .01) remained independent predictors of hematoma expansion, whereas the presence of hypodensities remained an independent NCCT predictor with the greatest nominal OR (adjusted OR, 3.07 [95% CI, 1.91–4.97]; P < .001).
Replication Cohort
Similar results were found in the replication cohort, with hypodensities being a strong independent predictor of expansion in the multivariable model (adjusted OR, 4.37 [95% CI, 2.05–9.62]; P < .001).
Spatial Correlation of Hypodensities and Spot Sign
As an exploratory analysis, a subset of 40 patients with both hypodensities and spot sign, randomly selected from each cohort (20 patients each) were specifically analyzed to assess the spatial correlation between both. The spot signs were spatially correlated with a hypodense area in 14 patients (35.0%) and not in 26 patients (65.0%) (eFigure 2 in the Supplement). Thirty-eight patients (95.0%) had at least 1 additional hypodensity not spatially matching the spot sign. Having spatially correlated spots and hypodensities was not associated with different rates of hematoma expansion in this subset of patients (data not shown).
Discussion
The presence of hypodensities within the hematoma detected by NCCT was independently associated with hematoma expansion. Because NCCT is widely available and commonly performed for acute ICH, this finding can be of prognostic significance and may help target anti-expansion therapies to those most likely to benefit. Furthermore, even in the setting of CTA and spot sign detection, hypodensities add substantial independent predictive value to assessing the risk of hematoma expansion.
Numerous groups have examined the value of NCCT in predicting expansion.26,27,36–38 While many have described different specific findings, we found variable interrater reliability in detecting specific features. However, we found that marking “any hypodensity” was both consistent and predictive, suggesting that all groups (including ourselves) may be examining different manifestations of the same phenomenon. Consistent with this, hypodensities were more likely to be present in larger hematomas, in patients scanned early, in those anticoagulated, with spot signs and intraventricular extension, all conditions known to be associated with a more severe presentation and a worse clinical outcome.
It is not currently clear what hypodensities represent, or perhaps what the heterogeneity27 of the hematomas represents. Shorter time to CT had an independent effect on the presence of hypodensities after adjustment for potential cofounders in both cohorts, which suggests that hypodensities mark those hematomas in an earlier stage of development.
Hematoma growth may occur in a cascaded fashion, with initial bleeding causing secondary mechanical shearing of peripheral vessels responsible for ongoing (and most likely sequential) bleeding.39–43 It may be that intra-hematoma heterogeneity reflects different ongoing phases of bleeding and thus marks a hematoma with sites prone to further rupture and expansion. Our exploratory analysis of the spatial correlation between CTA spots and hypodensities suggests that these 2 findings may mark different processes, which still remain to be elucidated.
We do note that our initial plan had been to examine multiple prespecified hypodensity subtypes. In the course of our initial analysis, however, we found that maximal interrater reliability and reproducibility came from simply dichotomizing NCCT scans into the presence or absence of any hypodensity. In addition, there was no clear signal that different subtypes contributed differentially to hematoma expansion. Therefore, our analysis plan was adjusted to simply mark whether any hypodensity was present, and this dichotomization was used in the replication cohort. We noted that the rate of hematoma expansion was slightly higher in the replication cohort (61 of 245 patients [24.9%]) than in the development cohort (163 of 784 patients [20.8%]) (P = .06). It may be that over time, referral patterns have changed such that higher risk patients are disproportionately transferred to our center. In support of this possibility, the patients in the replication cohort had larger ICH volumes, shorter times to CT, and more frequent spot signs. It also may be that, owing to increasing public awareness, patients are presenting earlier in their disease course.
At the moment, various clinical trials are underway to determine the value of anti-expansion strategies.7,10,13,44,45 In some, the CTA spot sign is being used to select patients for therapy.45 While a strong predictor, CTA is not available or actively used at all hospitals in the acute setting.46 Therefore, there is a clear role for the use of an NCCT finding in predicting expansion. It may be that, when CTA is not available, NCCT findings such as hypodensities can be used to select therapies such as blood pressure reduction, hemostatic therapy, or specific anticoagulation reversal strategies just for those patients at highest risk. In addition, these findings may help us select levels of care for patients with ICH; it may be that when resources are limited, patients can be stratified to regular stroke unit care vs intensive care based on their risk of expansion and deterioration. Moreover, even when CTA is readily available, assessing the presence of hypodensities and spot signs provides a more accurate predictive value than either one alone, given their independent association with expansion.
The strengths of this study are its large sample size, the prospectively acquired clinical data, and the replication of the findings in an independent cohort. Despite the potential change in clinical practice over the study period, the ability of hypodensities to predict expansion remained independent in both cohorts.
Moreover, while ICH care may have changed during the study period,47–49 hypodensities consistently remained a strong predictor of expansion, further strengthening the applicability of our results.
The limitations of this study include that it is a single-center cohort study, and so the findings will require replication in other centers. In addition, as an observational study, clinical care may have varied, and imaging acquisition was not consistently performed at specified time points. Indeed, early in-hospital mortality or withdrawal of care often precluded the acquisition of a follow-up scan, particularly for patients with larger hematomas or receiving anticoagulation and thus at potentially higher risk of expansion.
Conclusions
In conclusion, the presence of hypodensities within the hematoma may predict hematoma expansion in the first 48 hours after ICH. These findings add substantially to our ability to predict the risk of hematoma expansion in clinical practice and to our understanding of the dynamic intra-hematoma processes that indicate ongoing bleeding. Advances in both areas will be key to future interventional trials aimed at improving ICH outcomes.
Supplementary Material
Key Points.
Question
Do hypodense regions within an acute intracerebral hemorrhage, detected by noncontrast computed tomography, independently predict hematoma expansion?
Findings
This large cohort study that included 1029 patients demonstrates that hypodensities within an acute intracerebral hemorrhage independently predict hematoma expansion.
Meaning
The presence of hypodensities on noncontrast computed tomographic scans may help guide the management of patients with an acute intracerebral hemorrhage.
Acknowledgments
Funding/Support: This work was supported by National Institutes of Health grants R01-AG026484 (Dr Greenberg) and R01-NS073344 (Dr Goldstein). Dr Boulouis was supported by a J. William Fulbright Scholarship and a Monahan Foundation Biomedical Research Grant.
Footnotes
Conflict of Interest Disclosures: Dr Goldstein has received consulting and research contracts from CSL Behring and Boehringer Ingelheim. No other disclosures were reported.
Author Contributions: Dr Boulouis had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Boulouis, Morotti, Brouwers, Auriel, Pontes-Neto, Goldstein.
Acquisition, analysis, or interpretation of data: Boulouis, Morotti, Brouwers, Charidimou, Jessel, Ayres, Vashkevich, Schwab, Rosand, Viswanathan, Gurol, Greenberg, Goldstein.
Drafting of the manuscript: Boulouis, Goldstein.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Boulouis, Morotti, Jessel.
Obtained funding: Rosand, Viswanathan, Greenberg, Goldstein.
Administrative, technical, or material support: Boulouis, Jessel, Vashkevich, Schwab, Goldstein.
Study supervision: Boulouis, Viswanathan, Goldstein.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publications.
References
- 1.Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344(19):1450–1460. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- 2.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9(2):167–176. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- 3.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage: a powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24(7):987–993. doi: 10.1161/01.str.24.7.987. [DOI] [PubMed] [Google Scholar]
- 4.Flaherty ML, Haverbusch M, Sekar P, et al. Long-term mortality after intracerebral hemorrhage. Neurology. 2006;66(8):1182–1186. doi: 10.1212/01.wnl.0000208400.08722.7c. [DOI] [PubMed] [Google Scholar]
- 5.Davis SM, Broderick J, Hennerici M, et al. Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006;66(8):1175–1181. doi: 10.1212/01.wnl.0000208408.98482.99. [DOI] [PubMed] [Google Scholar]
- 6.Brouwers HB, Greenberg SM. Hematoma expansion following acute intracerebral hemorrhage. Cerebrovasc Dis. 2013;35(3):195–201. doi: 10.1159/000346599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayer SA, Brun NC, Begtrup K, et al. FAST Trial Investigators. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358(20):2127–2137. doi: 10.1056/NEJMoa0707534. [DOI] [PubMed] [Google Scholar]
- 8.Anderson CS, Heeley E, Huang Y, et al. INTERACT2 Investigators. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368(25):2355–2365. doi: 10.1056/NEJMoa1214609. [DOI] [PubMed] [Google Scholar]
- 9.Delcourt C, Huang Y, Wang J, et al. INTERACT2 Investigators. The second (main) phase of an open, randomised, multicentre study to investigate the effectiveness of an intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT2) Int J Stroke. 2010;5(2):110–116. doi: 10.1111/j.1747-4949.2010.00415.x. [DOI] [PubMed] [Google Scholar]
- 10.Qureshi AI, Palesch YY, Martin R, et al. Interpretation and Implementation of Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage Trial (INTERACT II) J Vasc Interv Neurol. 2014;7(2):34–40. [PMC free article] [PubMed] [Google Scholar]
- 11.Mayer SA, Davis SM, Skolnick BE, et al. FAST trial investigators. Can a subset of intracerebral hemorrhage patients benefit from hemostatic therapy with recombinant activated factor VII? Stroke. 2009;40(3):833–840. doi: 10.1161/STROKEAHA.108.524470. [DOI] [PubMed] [Google Scholar]
- 12.Qureshi AI, Palesch YY. Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) II: design, methods, and rationale. Neurocrit Care. 2011;15(3):559–576. doi: 10.1007/s12028-011-9538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qureshi AI, Palesch YY, Martin R, et al. Antihypertensive Treatment of Acute Cerebral Hemorrhage Study Investigators. Effect of systolic blood pressure reduction on hematoma expansion, perihematomal edema, and 3-month outcome among patients with intracerebral hemorrhage: results from the antihypertensive treatment of acute cerebral hemorrhage study. Arch Neurol. 2010;67(5):570–576. doi: 10.1001/archneurol.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Arima H, Al-Shahi Salman R, et al. INTERACT Investigators. Clinical prediction algorithm (BRAIN) to determine risk of hematoma growth in acute intracerebral hemorrhage. Stroke. 2015;46(2):376–381. doi: 10.1161/STROKEAHA.114.006910. [DOI] [PubMed] [Google Scholar]
- 15.Huynh TJ, Aviv RI, Dowlatshahi D, et al. PREDICT/Sunnybrook CTA Investigators. Validation of the 9-Point and 24-Point Hematoma Expansion Prediction Scores and Derivation of the PREDICT A/B Scores. Stroke. 2015;46(11):3105–3110. doi: 10.1161/STROKEAHA.115.009893. [DOI] [PubMed] [Google Scholar]
- 16.Brouwers HB, Chang Y, Falcone GJ, et al. Predicting hematoma expansion after primary intracerebral hemorrhage. JAMA Neurol. 2014;71(2):158–164. doi: 10.1001/jamaneurol.2013.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brouwers HB, Battey TWK, Musial HH, et al. Rate of contrast extravasation on computed tomographic angiography predicts hematoma expansion and mortality in primary intracerebral hemorrhage. Stroke. 2015;46(9):2498–2503. doi: 10.1161/STROKEAHA.115.009659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wada R, Aviv RI, Fox AJ, et al. CT angiography “spot sign” predicts hematoma expansion in acute intracerebral hemorrhage. Stroke. 2007;38(4):1257–1262. doi: 10.1161/01.STR.0000259633.59404.f3. [DOI] [PubMed] [Google Scholar]
- 19.Demchuk AM, Dowlatshahi D, Rodriguez-Luna D, et al. PREDICT/Sunnybrook ICH CTA study group. Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the CT-angiography spot sign (PREDICT): a prospective observational study. Lancet Neurol. 2012;11(4):307–314. doi: 10.1016/S1474-4422(12)70038-8. [DOI] [PubMed] [Google Scholar]
- 20.Kim J, Smith A, Hemphill JC, III, et al. Contrast extravasation on CT predicts mortality in primary intracerebral hemorrhage. AJNR Am J Neuroradiol. 2008;29(3):520–525. doi: 10.3174/ajnr.A0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barras CD, Tress BM, Christensen S, et al. Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Density and shape as CT predictors of intracerebral hemorrhage growth. Stroke. 2009;40(4):1325–1331. doi: 10.1161/STROKEAHA.108.536888. [DOI] [PubMed] [Google Scholar]
- 22.Selariu E, Zia E, Brizzi M, Abul-Kasim K. Swirl sign in intracerebral haemorrhage: definition, prevalence, reliability and prognostic value. BMC Neurol. 2012;12(1):109. doi: 10.1186/1471-2377-12-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galbois A, Boëlle PY, Hainque E, et al. Prediction of evolution toward brain death upon admission to ICU in comatose patients with spontaneous intracerebral hemorrhage using simple signs. Transpl Int. 2013;26(5):517–526. doi: 10.1111/tri.12084. [DOI] [PubMed] [Google Scholar]
- 24.Gökçe E, Beyhan M, Acu B. Evaluation of oral anticoagulant-associated intracranial parenchymal hematomas using CT findings. Clin Neuroradiol. 2015;25(2):151–159. doi: 10.1007/s00062-014-0292-8. [DOI] [PubMed] [Google Scholar]
- 25.Al-Nakshabandi NA. The swirl sign. Radiology. 2001;218(2):433. doi: 10.1148/radiology.218.2.r01fe09433. [DOI] [PubMed] [Google Scholar]
- 26.Barras CD, Tress BM, Christensen S, et al. Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Quantitative CT densitometry for predicting intracerebral hemorrhage growth. AJNR Am J Neuroradiol. 2013;34(6):1139–1144. doi: 10.3174/ajnr.A3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blacquiere D, Demchuk AM, Al-Hazzaa M, et al. PREDICT/Sunnybrook ICH CTA Study Group. Intracerebral hematoma morphologic appearance on noncontrast computed tomography predicts significant hematoma expansion. Stroke. 2015;46(11):3111–3116. doi: 10.1161/STROKEAHA.115.010566. [DOI] [PubMed] [Google Scholar]
- 28.Li Q, Zhang G, Huang YJ, et al. Blend sign on computed tomography novel and reliable predictor for early hematoma growth in patients with intracerebral hemorrhage. Stroke. 2015;46(8):2119–2123. doi: 10.1161/STROKEAHA.115.009185. [DOI] [PubMed] [Google Scholar]
- 29.Connor D, Huynh TJ, Demchuk AM, et al. Swirls and spots: relationship between qualitative and quantitative hematoma heterogeneity, hematoma expansion, and the spot sign. Neurovascular Imaging. 2015;1:8. doi: 10.1186/s40809-015-0010-1. [DOI] [Google Scholar]
- 30.Brouwers HB, Raffeld MR, van Nieuwenhuizen KM, et al. CT angiography spot sign in intracerebral hemorrhage predicts active bleeding during surgery. Neurology. 2014;83(10):883–889. doi: 10.1212/WNL.0000000000000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldstein JN, Fazen LE, Snider R, et al. Contrast extravasation on CT angiography predicts hematoma expansion in intracerebral hemorrhage. Neurology. 2007;68(12):889–894. doi: 10.1212/01.wnl.0000257087.22852.21. [DOI] [PubMed] [Google Scholar]
- 32.Delgado Almandoz JE, Yoo AJ, Stone MJ, et al. Systematic characterization of the computed tomography angiography spot sign in primary intracerebral hemorrhage identifies patients at highest risk for hematoma expansion: the spot sign score. Stroke. 2009;40(9):2994–3000. doi: 10.1161/STROKEAHA.109.554667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dowlatshahi D, Demchuk AM, Flaherty ML, Ali M, Lyden PL, Smith EE VISTA Collaboration. Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology. 2011;76(14):1238–1244. doi: 10.1212/WNL.0b013e3182143317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang W, Hu J, Zhang H, Wu P, He H. Kappa coefficient: a popular measure of rater agreement. Shanghai Arch Psychiatry. 2015;27(1):62–67. doi: 10.11919/j.issn.1002-0829.215010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 36.Yao X, Xu Y, Siwila-Sackman E, Wu B, Selim M. The HEP score: a nomogram-derived hematoma expansion prediction scale. Neurocrit Care. 2015;23(2):179–187. doi: 10.1007/s12028-015-0147-4. [DOI] [PubMed] [Google Scholar]
- 37.Chan S, Conell C, Veerina KT, Rao VA, Flint AC. Prediction of intracerebral haemorrhage expansion with clinical, laboratory, pharmacologic, and noncontrast radiographic variables. Int J Stroke. 2015;10(7):1057–1061. doi: 10.1111/ijs.12507. [DOI] [PubMed] [Google Scholar]
- 38.Witsch J, Bruce E, Meyers E, et al. Intraventricular hemorrhage expansion in patients with spontaneous intracerebral hemorrhage. Neurology. 2015;84(10):989–994. doi: 10.1212/WNL.0000000000001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fisher CM. Pathological observations in hypertensive cerebral hemorrhage. J Neuropathol Exp Neurol. 1971;30(3):536–550. doi: 10.1097/00005072-197107000-00015. [DOI] [PubMed] [Google Scholar]
- 40.Fisher CM. Hypertensive cerebral hemorrhage. Demonstration of the source of bleeding. J Neuropathol Exp Neurol. 2003;62(1):104–107. doi: 10.1093/jnen/62.1.104. [DOI] [PubMed] [Google Scholar]
- 41.Greenberg CH, Frosch MP, Goldstein JN, Rosand J, Greenberg SM. Modeling intracerebral hemorrhage growth and response to anticoagulation. PLoS One. 2012;7(10):e48458. doi: 10.1371/journal.pone.0048458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boulouis G, Dumas A, Betensky RA, et al. Anatomic pattern of intracerebral hemorrhage expansion relation to CT angiography spot sign and hematoma center. Stroke. 2014;45(4):1154–1156. doi: 10.1161/STROKEAHA.114.004844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dowlatshahi D, Wasserman JK, Momoli F, et al. Ottawa Stroke Research Group. Evolution of computed tomography angiography spot sign is consistent with a site of active hemorrhage in acute intracerebral hemorrhage. Stroke. 2014;45(1):277–280. doi: 10.1161/STROKEAHA.113.003387. [DOI] [PubMed] [Google Scholar]
- 44.Anderson CS, Huang Y, Wang JG, et al. INTERACT Investigators. Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): a randomised pilot trial. Lancet Neurol. 2008;7(5):391–399. doi: 10.1016/S1474-4422(08)70069-3. [DOI] [PubMed] [Google Scholar]
- 45.Goldstein J, Brouwers H, Romero J, et al. SCORE-IT: the Spot Sign score in restricting ICH growth—an Atach-II ancillary study. J Vasc Interv Neurol. 2012;5(1.5):20–25. [PMC free article] [PubMed] [Google Scholar]
- 46.Menon BK, Demchuk AM. Computed tomography angiography in the assessment of patients with stroke/TIA. Neurohospitalist. 2011;1(4):187–199. doi: 10.1177/1941874411418523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morgenstern LB, Hemphill JC, III, Anderson C, et al. American Heart Association Stroke Council and Council on Cardiovascular Nursing. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41(9):2108–2129. doi: 10.1161/STR.0b013e3181ec611b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steiner T, Al-Shahi Salman R, Beer R, et al. European Stroke Organisation. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke. 2014;9(7):840–855. doi: 10.1111/ijs.12309. [DOI] [PubMed] [Google Scholar]
- 49.Hemphill JC, 3rd, Greenberg SM, Anderson CS, et al. American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(7):2032–2060. doi: 10.1161/STR.0000000000000069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.