Abstract
Human airway basal cells (BC) function as stem/progenitor cells of the human airway epithelium, capable of differentiating into ciliated and secretory cells during turnover and repair. The positioning of BC along the basement membrane allows for potential paracrine signaling from non-epithelial cells in the mesenchyme to regulate BC function. Based on the knowledge that interaction between the airway epithelium and mesenchyme is critical for proper maintenance of both tissues, and that endothelial cells (EC) can regulate multiple functions of BC, the present study was designed to help understand the role of BC and EC cross-talk in regulating BC stem/progenitor function. Using an in vitro co-culture system that mimics the in vivo physical separation of these cell types, we assessed the impact of primary lung microvascular EC on differentiation of primary BC into a mucociliated epithelium. The data demonstrate that co-culture of BC and lung microvasculature EC results in increased ciliated cell differentiation of BC via activation of insulin (INS) and insulin-like growth factor 1 (IGF1) receptor (INSR and IGF1R) mediated signaling in BC. Consistent with this data, siRNA mediated knockdown of INSR and IGF1R in BC suppressed ciliated cell differentiation. Together these findings identify an important signaling pathway required for differentiation of BC into a ciliated cells and demonstrate the importance of BC-EC cross-talk in regulating normal airway epithelial structure.
Introduction
The human airway epithelium is a complex tissue that covers the surface of the respiratory tree and acts as a barrier to protect the lung from pathogens, irritants, toxins and other harmful environmental factors [1–3]. The major cell populations of the normal airway epithelium include ciliated, secretory, basal and intermediate cells, with each cell population having a specific role related to the function of the airway epithelium [1–3]. The luminal ciliated and secretory cells contribute to removal of foreign particles and help in the overall defense of the airway [4]. Basal cells (BC) reside in the basal epithelial layer immediately above the basement membrane and function as the stem/progenitor population of the human airway epithelium capable of differentiating into ciliated and secretory cells via a multi-step process involving BC-derived undifferentiated intermediate cell progenitors [5–14].
The anatomical positioning of BC along the basement membrane allows for potential paracrine signaling from non-epithelial cell types in the underlying mesenchyme [2, 3, 11]. Based on the knowledge that interaction between the airway epithelium and mesenchyme contributes to the proper maintenance of both tissues, understanding the cross-talk between airway BC and mesenchymal populations is important to understanding the processes that regulate maintenance of normal airway epithelial structure [15–17]. Endothelial cells (EC) in the airway vasculature are an important cell population of the mesenchyme and previous studies have demonstrated reciprocal cross-talk/signaling between EC and human BC to regulate multiple functions of BC including proliferation and differentiation into bronchioalveolar-like structures, suggesting EC are capable of modulating the stem/progenitor functions of BC [18–20].
The present study was designed to further understand the role of BC and EC cross-talk in regulating BC stem/progenitor functions with a specific focus on the role of EC-derived signals in regulating BC differentiation into a mucociliated epithelium. Using an in vitro co-culture system that mimics the in vivo physical separation of these cell types, we assessed the impact of primary lung microvascular EC on differentiation of primary BC into a mucociliated epithelium. The data demonstrate that co-culture of BC and lung microvasculature EC results in increased ciliated cell differentiation of BC via activation of insulin (INS) and insulin-like growth factor 1 (IGF1) receptor (INSR and IGF1R) mediated signaling in BC. Consistent with this concept, suppression of INSR and IGF1R signaling via siRNA mediated knockdown of each receptor in BC suppresses ciliated differentiation.
Methods
Culture of Primary Human Airway Basal Cells
Nonsmoker primary airway basal cells (BC) were obtained from Lonza (CC2540S, Walkersville, MD). In total, n=6 independent donors were used with the following demographics: donor 1 (male, Hispanic, 64 years old), donor 2 (female, African American, 56 years old), donor 3 (male, Caucasian, 56 years old), donor 4 (female, Hispanic, 44 years old), donor 5 (female, Caucasian, 69 years old) and donor 6 (female, Caucasian, 57 years old). All cultures were seeded at 3000 cells/cm2 into plastic flasks and maintained in Bronchial Epithelial Growth Media (BEGM, Lonza) [21]. Once the cells had reached 80% confluence, the cells were harvested for air-liquid interface (ALI) culture based experiments including co-culture with primary human lung microvasculature endothelial cells or siRNA mediated knockdown of specific genes.
Culture of Primary Human Lung Microvascular Endothelial cells
Nonsmoker primary lung microvascular endothelial cells (EC) were obtained from Lonza (CC-2527). In total, n=5 independent donors were used with the following demographics: donor 1 (female, Caucasian, 66 years old), donor 2 (female, African American, 46 years old), donor 3 (female, Hispanic, 61 years old), donor 4 (male, Caucasian, 14 years old) and donor 5 (female, Hispanic, 69 years old). All cultures were seeded at 3000 cells/cm2 into flasks pre-coated with fibronectin (F0895, Sigma, St Louis, MO) and maintained in Microvascular Endothelial Growth Medium-2 (EGM-2MV, Lonza). Once the cells had reached 80% confluence, the cells were harvested for co-culture with primary human airway BC as described below.
Co-culture of BC and EC
To investigate the impact of lung microvascular EC on differentiation of airway BC a co-culture system was developed based on our standard air-liquid interface (ALI) culture method [21, 22]. Briefly, the upper surface of 0.4 μm pore-sized Transwell inserts (Corning Incorporated, Corning, NY, USA) was pre-coated with type IV collagen (C7521, Sigma) and the lower surface with fibronectin (F0895, Sigma). Following coating, EC were seeded at two different densities (3.75 × 104 cells/cm2, BC:EC ratio 10:1 and 1.87 × 105 cells/cm2, BC:EC ratio 2:1) onto the lower surface (fibronectin coated) of the inverted Transwell inserts in 100 μl of EGM-2MV media. Two hr post seeding, the Transwell insert was gently reversed and 1ml of EGM-2MV media added into the chamber and incubated overnight (referred to as ALI day -3). The next day, the EGM-2MV media was removed from the lower chamber and replaced with culture medium consisting of a 1:1 mixture of DMEM (Cellgro, Manassas, VA, USA) and Ham’s F-12 Nutrient Mix (GIBCO-Invitrogen, Carlsbad, CA, USA) containing 5% fetal bovine serum, 1% penicillin-streptomycin, 0.1% gentamycin and 0.5% amphotericin B. Subsequently BC were seeded onto the upper surface of the Transwell insert with the same media at a density of 3.75 × 105 cells/cm2 (ALI day −2). The following day, the medium was changed to 1:1 DMEM/Ham’s F12 including antibiotics described above with 2% Ultroser G serum substitute (BioSerpa S.A., Cergy-Saint-Christophe, France). Once the BC reached confluence (following 2 days of culture), the media was removed from the upper chamber to expose the apical surface to air and establish the ALI (ALI day 0). Fresh culture media was replaced in the lower chamber every 2 days until the desired time of harvest. For the duration of the experiment, cells were incubated at 37°C, 8% CO2 for ALI day −3 to 5, then 37°C, 5% CO2 until harvest. To analyze BC differentiation at the molecular level using TaqMan PCR to assess mRNA expression of cell type specific markers and to assess activity of specific kinase signaling pathways, the BC were trypsinized and removed from the Tanswell inserts to separate them from the EC before processing for each specific assay as described below. For histological analysis of BC differentiation, ALI transwell inserts were fixed for paraffin embedding and sectioning (performed by Histoserv, Germantown, MD) at specific time points.
RNA Extraction, cDNA Synthesis and TaqMan PCR
Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA) and purified using the Rneasy MinElute RNA purification kit (Qiagen, Valencia, CA). The concentration of extracted RNA was determined using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). Double-stranded cDNA was synthesized from 1 μg of total RNA using Taq-Man Reverse Transcription Reagents (Applied Biosystems, Foster City, CA). The expression of specific genes was assessed using TaqMan quantitative PCR and relative expression levels determined using the dCt method with 18S ribosomal RNA (Cat. No: 4308329, ThermoFisher Scientific, Waltham, MA) as the endogenous control [21, 22]. Premade gene assays were obtained from ThermoFisher Scientific: KRT5 (Hs00361185_m1); KRT8 (Hs01595539_g1); SCGB1A1 (Hs00171092_m1); MUC5B (Hs00861588_m1); FOXJ1 (Hs00230964_m1); MYB (Hs00920556_m1); DNAI1 (Hs00201755_m1); INS (Hs00355773_m1); IGF1 (Hs01547656_m1); IGF2 (Hs04188276_m1); INSR (Hs00961560_m1) and IGF1R (Hs00609566_m1).
Immunofluorescence, Quantification of Differentiation
Immunofluorescent staining was performed on either paraffin embedded cross-sections of normal human bronchus tissue (Cat No: HuFPT111, US Biomax, Inc., Rockville, MD), paraffin embedded cross-sections of ALI cultures or direct top-staining of the ALI membrane [21, 22]. The samples were then stained with the following primary antibodies: rabbit monoclonal CD31 (5 μg/ml; ab76533; Abcam, Cambridge, UK); goat polyclonal VE-Cadherin (1 μg/ml; AF938; R&D Systems Inc., Minneapolis, MN), mouse monoclonal KRT5 (4 μg/ml; MA5-12596; ThermoFisher Scientific); rabbit polyclonal KRT5 (2 μg/ml; PA1-37974; ThermoFisher Scientific); rabbit polyclonal KRT8 (10 μg/ml; NBP2-16094; Novus Biological, Littleton, CO); rabbit polyclonal SCGB1A1 (5 μg/ml; RD181022220; BioVendor LLC, Candler, NC); rabbit polyclonal MUC5B (4 μg/ml; sc-20119; Santa Cruz Biotechnology, Santa Cruz, CA) and mouse monoclonal β-tubulin IV (10 μg/ml; MU178-UC; Biogenex, Fremont, CA) at 4°C overnight. Isotype matched IgG (Jackson Immunoresearch Laboratories, West Grove, PA) was the negative control. To visualize the antibody binding, Alexa Fluor® 488 Goat Anti-Mouse IgG (A-11029), Alexa Fluor® 546 Goat Anti-Rabbit IgG (A-11035), Alexa Fluor® 488 Donkey Anti-Goat IgG (A-11055) and Alexa Fluor® 555 Donkey Anti- Rabbit IgG (A-31572; all from Life Technologies, Carlsbad, CA) labeled secondary antibodies were used. The cells were counterstained with DAPI to identify cell nuclei and subsequently mounted using ProLong® Gold antifade reagent (Invitrogen).
For quantification of BC differentiation at the histological level via immunofluorescence staining using cell type specific antibodies, a minimum of 10 images equally distributed between both ends of the sectioned membrane were acquired and a minimum of 200 total cells counted for each individual experiment. Basal cells were determined by positivity for the marker KRT5, intermediate cells by positivity for the marker KRT8, secretory cells by positivity for the markers SCGB1A1 or MUC5B and ciliated cells were determined by positivity for β-tubulin IV.
Kinase Signaling Pathway Analysis
To assess the activity of specific kinase signaling pathways in BC in response to EC derived factors during co-culture, the PathScan RTK signaling array kit containing 28 fixed antibodies against phosphorylated forms of receptor tyrosine kinases and 11 signaling nodes was used (7982, Cell Signaling Technologies, Danvers, MA). Basal cells were cultured alone or co-cultured with EC at a 10:1 (BC:EC) ratio on ALI culture for 7 days as described above and the cell lysates from BC cultured in each condition were generated using the manufacturers protocol. Subsequently 100 μg of total protein for each sample was run on the array and processed using the manufacturer’s protocol. The intensities of the spots were quantified with ImageJ (http://rsbweb.nih.gov/ij/) and all values normalized by the positive control (100%) and negative control spots (0%) on the array.
siRNA Mediated Knockdown of Genes
Basal cells were transfected with 5 pmol of either control siRNA, insulin receptor (INSR) specific siRNA or insulin-like growth factor 1 receptor (IGF1R) specific siRNA (all from Life Technologies) using Lipofectamine RNAiMAX Reagent and Opti-MEM media (both from Life Technologies) at the time of seeding cells for ALI culture. The next day the standard protocol for ALI culture was continued.
Statistical Analysis
The Mann-Whitney U test was used for statistical analyses of all experiments. In all analyses, a p value less than 0.05 was deemed significant.
Results
Development of in vitro Basal and Endothelial Cell Co-culture System
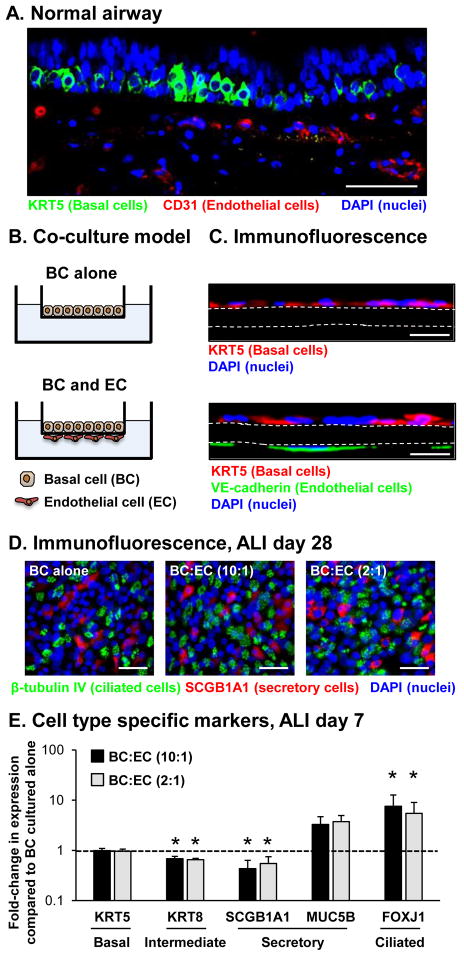
The positioning of BC along the basement membrane and their close proximity to capillary EC in the underlying mesenchymal layer allows for potential paracrine signaling between these two cell types (Figure 1A). To further investigate this potential paracrine signaling between BC and EC, with a specific focus on the role of EC derived factors in regulating BC differentiation into a mucociliated epithelium, an in vitro co-culture system was developed that mimics the in vivo physical separation of the cell types to assess the impact of lung microvascular EC on differentiation of BC (Figure 1B, C). Within this system, EC are seeded on the underside of the Transwell insert with BC seeded in the apical chamber with the membrane of the Transwell insert physically separating the two cell populations and mimicking the function of the basement membrane in vivo. The system allows for the differentiation of BC into a mucociliated epithelium following day 28 days of air-liquid interface (ALI culture) in the absence and presence of different ratios of EC (Figure 1D). To assess the impact of EC on the early stages of BC differentiation into a mucociliated epithelium, BC were cultured alone or co-cultured with EC at BC to EC ratios of 10:1 and 2:1 and then analyzed at ALI day 7 by assessing mRNA expression of cell type specific markers. The results demonstrated no significant difference in expression of the BC marker KRT5 [0.99 fold BC:EC (10:1) and 0.96 fold BC:EC (2:1) both p>0.3] or mucus-producing secretory cell marker MUC5B [3.29 fold BC:EC (10:1) and 3.73 fold BC:EC (2:1) both p>0.3] in BC co-cultured with EC compared to BC cultured alone (Figure 1E). However, there was a significant decrease in expression of the intermediate cell marker KRT8 [0.68 fold BC:EC (10:1) and 0.65 fold BC:EC (2:1) both p<0.003] and the non-mucus secretory cell marker SCGB1A1 [0.43 fold BC:EC (10:1) and 0.55 fold BC:EC (2:1) both p<0.05] in BC co-cultured with EC compared to BC cultured alone. In contrast to KRT8 and SCGB1A1, there was a significant increase in expression of the ciliated cell marker FOXJ1 in BC co-cultured with EC compared to BC cultured alone [7.57 fold BC:EC (10:1) and 5.47 fold BC:EC (2:1) both p<0.05]. Together, these data suggest that co-culture of BC with lung microvasculature EC modulates the stem/progenitor function of BC during the early stages of differentiation into a mucociliated epithelium with skewing of differentiation to a decrease in expression of the intermediate cell (KRT8) and non-mucus secretory cell (SCGB1A1) markers and a corresponding increase in expression of the ciliated cell marker FOXJ1.
Figure 1.
Establishment of basal cell (BC) and endothelial cell (EC) co-culture system to study BC differentiation into a mucociliated epithelium. A. Immunofluorescence assessment of a normal human airway biopsy with staining for BC (KRT5, green), EC (CD31, red) and nuclei (DAPI, blue). Scale bar 50 μm. B. Schematic representation of the BC and EC co-culture system to study the impact of EC on BC differentiation into a mucociliated epithelium. C. Immunofluorescence assessment of BC and EC co-culture with staining for BC (KRT5, red), EC (VE-cadherin, green) and nuclei (DAPI, blue). Upper panel, BC cultured alone. Lower panel, BC and EC co-culture. White dashed line outlines the membrane of the Transwell insert. Scale bar 20 μm. D. Immunofluorescence staining of the epithelial layer of ALI day 28 cultures. Basal cells were cultured alone or co-cultured with EC at a 10:1 and 2:1 (BC:EC) ratio and then stained for ciliated cells (β-tubulin IV, green), secretory cells (SCGB1A1, red) and nuclei (DAPI, blue). Scale bar 20 μm. E. Basal cells were cultured alone or co-cultured with EC at a 10:1 and 2:1 (BC:EC) ratio on air-liquid interface (ALI) culture for 7 days to assess the impact of EC on the early stages of BC differentiation into a mucociliated epithelium at the molecular level. TaqMan PCR analysis to assess mRNA expression of BC (KRT5), intermediate (KRT8), secretory (non-mucus producing, SCGB1A1 and mucus producing, MUC5B) and ciliated cell (FOXJ1) markers in BC cultured alone or co-cultured with EC cells at 10:1 and 2:1 (BC:EC) ratio. Bars indicate the mean for n=6 independent experiments each performed in triplicate and error bars indicate standard error of the mean. Asterisks (*) indicate p<0.05 compared to BC alone. The experiments for E were performed with n=4 independent donors of BC and EC.
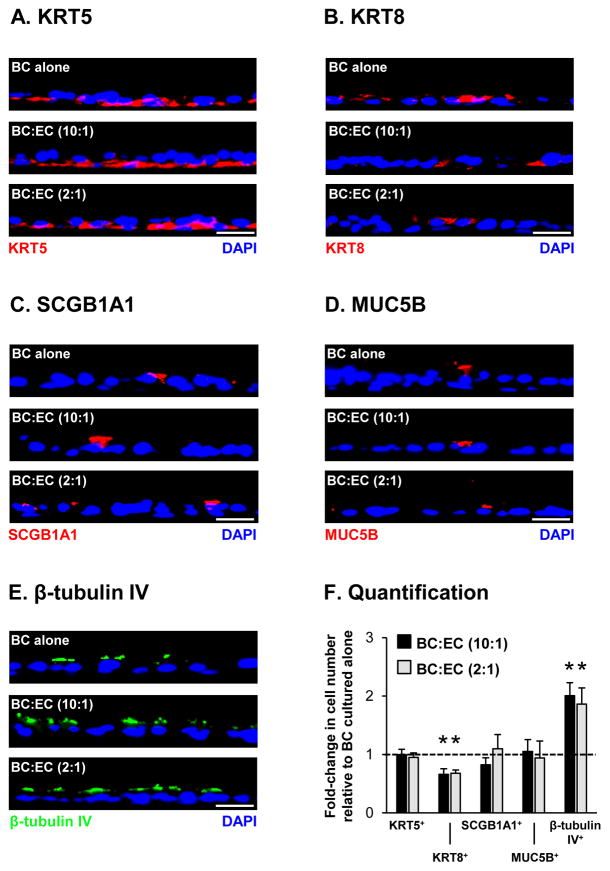
To further confirm these results and the effects of EC co-culture on terminal differentiation of BC into a mucociliated epithelium, we assessed differentiation at the histological level following 28 days on ALI culture by immunofluorescence staining of cell type specific markers (Figure 2). Compared to BC cultured alone, there was no significant difference in the numbers of KRT5+ BC with EC co-culture [1.00 fold BC:EC (10:1) and 0.95 fold BC:EC (2:1) both p>0.2, Figure 2A, F]. However, a significant decrease in the numbers of KRT8+ intermediate cells was observed with EC co-culture [0.66 fold BC:EC (10:1) and 0.68 fold BC:EC (2:1), both p<0.02, Figure 2B, F). No significant effect on the differentiation of BC into SCGB1A1+ [0.82 fold BC:EC (10:1) and 1.10 fold BC:EC (2:1) both p>0.2] and MUC5B+ [1.05 fold BC:EC (10:1) and 0.94 fold BC:EC (2:1) both p>0.2] secretory cells was observed with EC co-culture (Figure 2C, D, F). In contrast, EC co-culture significantly increased the number of β-tubulin IV+ ciliated cells compared to BC alone [2.01 fold BC:EC (10:1) and 1.86 fold BC:EC (2:1), both p<0.02, Figure 2E, F]. In summary, these data suggest that EC co-culture has no effect on the terminal differentiation of BC into secretory cells, but EC derived factors promote increased ciliated cell differentiation of BC-derived KRT8+ intermediate cells.
Figure 2.
Endothelial cell (EC) co-culture increases differentiation of basal cells (BC) into ciliated cells. Basal cells were cultured alone or co-cultured with EC at a 10:1 and 2:1 (BC:EC) ratio on air-liquid interface (ALI) culture for 28 days to assess the impact of EC on BC differentiation into a mucociliated epithelium at the histological level via immunofluorescence staining with cell type specific markers. A. KRT5+ BC. Sections of cells were stained for KRT5 (red) and DAPI (nuclei, blue). B. KRT8+ intermediate cells. Sections of cells were stained for KRT8 (red) and DAPI (nuclei, blue). C. SCGB1A1+ secretory cells. Sections of cells were stained for SCGB1A1 (red) and DAPI (nuclei, blue). D. MUC5B+ secretory cells. Sections of cells were stained for MUC5B (red) and DAPI (nuclei, blue). E β-tubulin IV+ cells. Sections of cells were stained for β-tubulin IV (ciliated, green) and DAPI (nuclei, blue). A–E. Scale bar 20 μm. H. Quantification of KRT5+, KRT8+, SCGB1A1+, MUC5B+ and β-tubulin IV+ cells. The bars indicate the mean for n=4 independent experiments and error bars indicate standard error of the mean. Asterisks (*) indicate p<0.05 compared to BC cultured alone. The experiments for A–H were performed with n=4 independent donors of BC and EC.
Endothelial Cell Co-culture Activates the Insulin and Insulin-like Growth Factor 1 Receptor Mediated Signaling Pathways in Basal Cells to Promote Ciliated Cell Differentiation
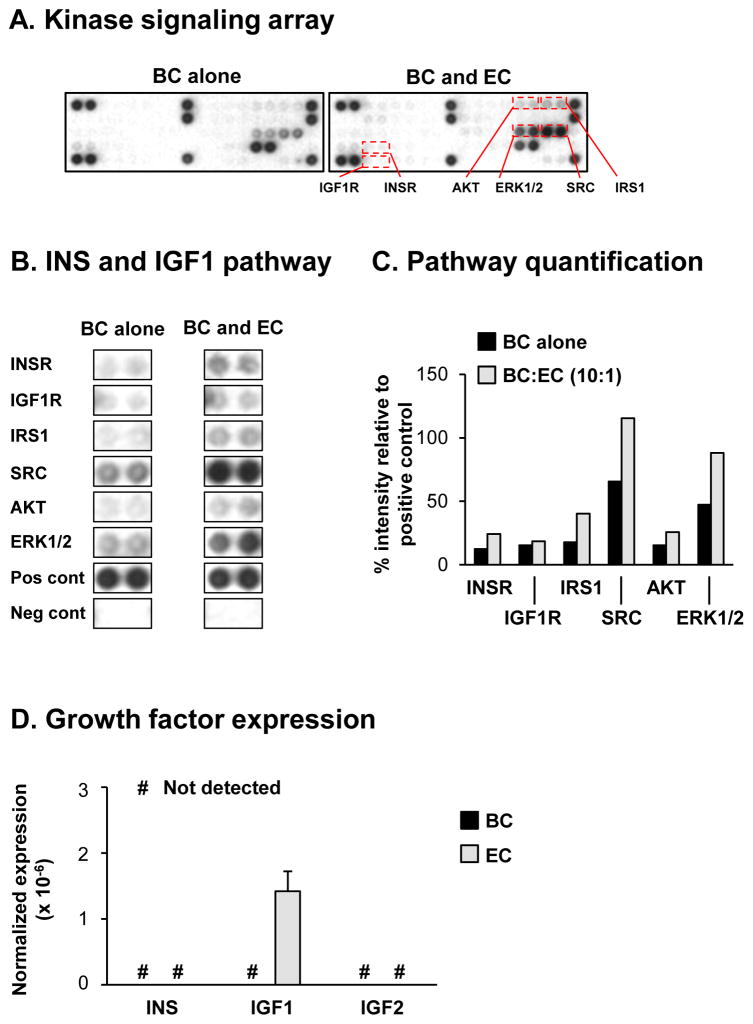
To identify specific signaling pathways in BC that promote ciliated cell differentiation in response to EC derived paracrine factors, we used a protein kinase signaling array containing antibodies against phosphorylated forms of multiple receptor tyrosine kinases and downstream signaling nodes. Based on the knowledge that EC co-culture significantly increases mRNA expression of the ciliated cell marker FOXJ1 at the early stages of BC differentiation (ALI day 7, Figure 1D), we hypothesized this time point would be suitable to identify specific signaling pathways that regulate ciliated cell differentiation. The study was focused on comparing the activity of multiple receptor tyrosine kinases and downstream signaling nodes in BC cultured alone vs BC co-cultured with EC at a ratio of 10:1 (BC:EC). The results demonstrated differences in the spot intensity of multiple proteins (indicative of altered phosphorylation and activation) in BC co-cultured with EC compared to BC cultured alone, with a large number of these proteins representative of receptor tyrosine kinases and signaling nodes involved in the insulin (INS) and insulin-like growth factor 1 (IGF1) signaling pathways (Figure 3A, B) [23–27]. Examples included the insulin receptor (INSR), insulin-like growth factor 1 receptor (IGF1R), insulin receptor substrate 1 (IRS1), SRC, AKT and ERK1/2. Quantification of spot intensities demonstrated increased phosphorylation (indicative of increased activity) in response to EC co-culture for INSR (12.4% BC alone vs 24.2% BC:EC co-culture), IGF1R (15.3% BC alone vs 18.5% BC:EC co-culture), IRS1 (17.8% BC alone vs 40.4% BC:EC co-culture), SRC (65.5% BC alone vs 115.4% BC:EC co-culture), AKT (15.5% BC alone vs 25.8% BC:EC co-culture) and ERK1/2 (47.3% BC alone vs 88.1% BC:EC co-culture, Figure 3C). Both INSR and IGF1R are upstream receptor tyrosine kinases that regulate activity of IRS1 and SRC, and subsequent activity of the major downstream effectors AKT and ERK1/2 suggesting activation of the INSR and IGF1R pathways. Quantitative PCR analysis of the growth factors (INS, IGF1 and IGF2) that activate these pathways in both BC and EC following co-culture demonstrated BC do not express INS, IGF1 or IGF2, whereas EC only express IGF1 (Figure 3D). Together, these data suggest that EC derived IGF1 functions in a paracrine manner to activate INSR and IGF1R mediated signaling in BC to promote ciliated differentiation.
Figure 3.
Signaling via the insulin (INS) and insulin-like growth factor 1 (IGF1) receptors (INSR and IGF1R) regulate basal cell (BC) differentiation into ciliated cells. A–C. Basal cells were cultured alone or co-cultured with EC at a 10:1 (BC:EC) ratio on air-liquid interface (ALI) culture for 7 days to assess the activity of specific kinase signaling pathways in BC in response to EC derived factors using the PathScan RTK Proteome Array (Cell signaling). A. Representative images from the same exposure times of the kinase signaling array from BC cultured alone and BC co-cultured with EC. B. Activity of the INS and IGF1 mediated signaling pathways including the receptor tyrosine kinases (ISNR and IGF1R) and downstream signaling nodes (IRS1, SRC, AKT and ERK1/2). “Pos cont” and “neg cont” represent the positive and negative control spots respectively on the array. C. Quantification of the INS and IGF1 signaling pathway activity. The intensities of spots were quantified with ImageJ and all values normalized by the positive control spots on the array (100%) and negative control spots (0%). D. TaqMan PCR analysis to assess mRNA expression of INS, IGF1 and IGF2 in BC (black bars) and EC (grey bars) following co-culture at ALI day 0. Bars indicate the mean expression for n=4 replicate wells and error bars indicate standard error of the mean.
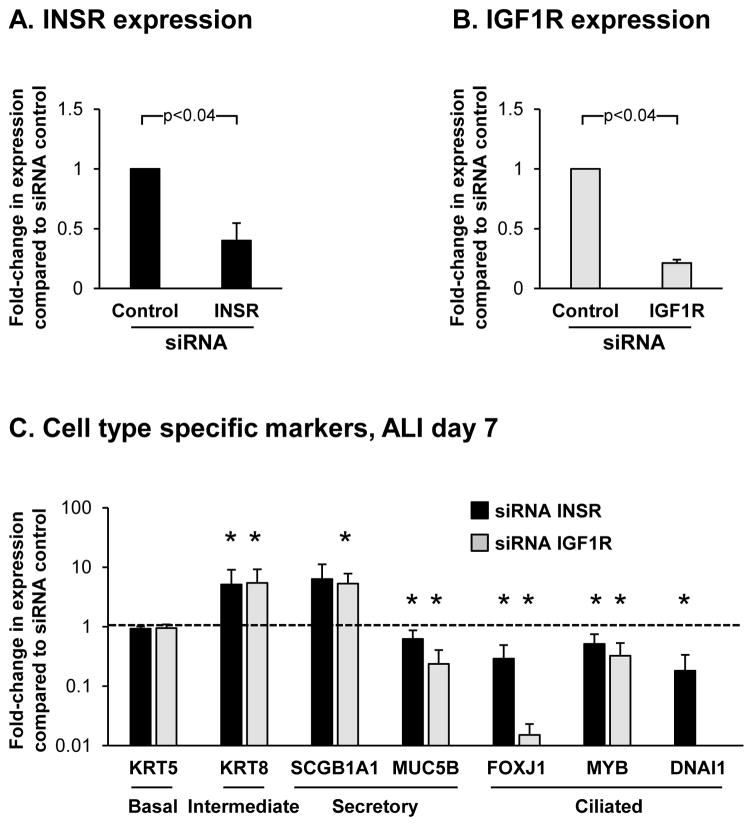
To further investigate the role of INSR and IGF1R mediated signaling in regulating BC differentiation into ciliated cells, we performed siRNA mediated knockdown of INSR and IGF1R in differentiating BC on ALI culture using specific siRNA for each receptor. Relative to control siRNA, quantitative PCR analysis confirmed knockdown of both INSR (0.40 fold, p<0.04) and IGF1R (0.21 fold, p<0.04) at the mRNA level in response to each specific siRNA (Figure 4A, B). Western analysis was performed to confirm the siRNA mediated knockdown of both INSR and IGF1R at the protein level. However, due to antibody sensitivity issues we were unable to detect either protein in lysates for the untransfected and siRNA control transfected cells (not shown). Therefore, currently we are unable to verify the siRNA knockdown of INSR and IGF1R at the protein level. To further characterize the effects of INSR and IGF1R knockdown on BC differentiation, the expression of cell type-specific markers were analyzed at ALI Day 7 (Figure 4C). Relative to control siRNA cells, knockdown of both INSR and IGF1R had no significant impact on expression of the BC marker KRT5 (0.9 fold, INSR and 1.0 fold IGF1R, both p>0.4), however there was a significant increase in expression of the intermediate cell marker KRT8 in response to knockdown of INSR and IGF1R (5.1 fold, INSR and 5.4 fold IGF1R, both p<0.04). A variable response of secretory cell marker expression was observed following INSR and IGFR1 knockdown with SCGB1A1 demonstrating increased expression (6.3 fold, INSR, p>0.4 and 5.3-fold IGF1R, p<0.04) and MUC5B demonstrating decreased expression (0.63 fold, INSR and 0.24-fold IGF1R, both p<0.04). In contrast, knockdown of both INSR and IGF1R resulted in a significant decrease in expression of the ciliated cell markers FOXJ1 (0.29 fold, INSR and 0.02 fold IGF1R, both p<0.04), MYB (0.51 fold, INSR and 0.33 fold IGF1R, both p<0.04) and DNAI1 (0.18 fold, INSR, p<0.04 and undetected for IGF1R). Interestingly, knockdown of IGF1R decreased expression of ciliated cell markers to a greater extent that INSR suggesting IGF1R mediated signaling has a greater role in regulating ciliated cell differentiation. However, this observation may result from the increased knockdown efficiency of IGF1R compared to INSR and future studies are required to verify this finding. Overall, the expression trends for the different cell type specific markers in response to knockdown of INSR and IGF1R were the opposite to those observed in BC during co-culture with EC at the same time point (Figure 1E), and further demonstrate that INSR and IGF1R mediated signaling regulate differentiation of BC into ciliated cells.
Figure 4.
siRNA mediated knockdown of INSR and IGF1R in basal cells (BC) suppresses ciliated cell differentiation. Basal cells with transfected with either control, INSR or IGF1R specific siRNA and cultured on ALI for 7 days to assess the impact of each receptor on the early stages of BC differentiation into a mucociliated epithelium at the molecular level. A. TaqMan PCR analysis to assess INSR mRNA expression at ALI day 0. Bars indicate the mean for n=3 independent experiments each performed in triplicate and error bars indicate standard error of the mean. B. TaqMan PCR analysis to assess IGF1R mRNA expression at ALI day 0. Bars indicate the mean for n=3 independent experiments each performed in triplicate and error bars indicate standard error of the mean. C. TaqMan PCR analysis to assess mRNA expression of BC (KRT5), intermediate (KRT8), secretory (non-mucus producing, SCGB1A1 and mucus producing, MUC5B) and ciliated cell markers (FOXJ1, MYB and DNAI1) in BC at ALI day 7 following siRNA mediated knockdown of INSR (black bars) and IGF1R (grey bars). Bars indicate the mean for n=3 independent experiments each performed in triplicate and error bars indicate standard error of the mean. Asterisks (*) indicate p<0.05 compared to BC transfected with control siRNA. The experiments for A–C were performed with n=3 independent donors of BC.
Discussion
Basal cells (BC) function as stem/progenitor cells of the human airway epithelium and differentiate into secretory and ciliated cells via a multi-step process to replenish the airway epithelium during normal turnover and repair [5–14]. The anatomical positioning of BC along the basement membrane allows for potential paracrine signaling from non-epithelial cell types in the underlying mesenchyme to regulate BC function [2, 3, 11]. Based on this concept, the present study was designed to further understand the role of cross-talk between BC and lung microvascular endothelial cells (EC) in regulating BC stem/progenitor functions using an in vitro co-culture system that mimics the in vivo physical separation of these cell types. The data demonstrate that co-culture of BC and lung microvasculature EC results in increased ciliated cell differentiation of BC, with a corresponding decrease in the number of KRT8+ intermediate cells, but has no effect on secretory cell numbers. Co-culture with EC increased activation of the insulin (INS) and insulin-like growth factor 1 (IGF1) receptor (INSR and IGF1R) mediated signaling pathways in BC and suppression of INSR and IGF1R signaling via siRNA mediated knockdown of each receptor in BC suppresses ciliated differentiation. Furthermore, gene expression analysis of INS, IGF1 and IGF2 growth factor ligands demonstrated BC lack expression of each ligand whereas EC only express IGF1 suggesting that EC-derived IGF1 functions in a paracrine manner to regulate ciliated cell differentiation of BC via activation of these signaling pathways.
The insulin and insulin-growth factor signaling pathways are complex signaling networks that play a critical role in regulating many cellular process including proliferation, survival and differentiation in diverse array of cell types [23–27]. The pathways consist of three receptor tyro-sine kinases (INSR, IGF1R and IGF2R) of which only INSR and IGF1R are capable of intracellular signaling activity in response to ligand binding. Activation of signaling is mediated by three ligands (INS, IGF1 and IGF2) which exert their effects through autocrine and paracrine mechanisms and six insulin-like growth factor binding proteins (IGFBP) that regulate activity of the pathways by determining bioavailability of the ligands. Prior studies have demonstrated a critical role for this pathway in regulating lung morphogenesis and distal lung differentiation in the murine developing lung, with suppression of IGF1/IGF1R signaling resulting in collapsed air spaces, distal septa remodeling along, altered epithelial differentiation and vascular maturation [28–30]. In support of our observations, Galvis et al [31] demonstrated that the histone methyltransferase Ezh2 regulates BC differentiation in the developing lung via repression of IGF1 expression. Conditional knockout of Ezh2 results in upregulation of IGF1 expression which in turn promotes BC differentiation resulting in increased ciliated cell differentiation in the proximal airways suggesting IGF1 mediated signaling regulates ciliated cell differentiation of BC. In addition to the murine lung, INS/IGF1 signaling also plays a critical role in regulating differentiation of murine epidermal BC to maintain normal stratification suggesting a conserved function of INSR and IGF1R mediated signaling in regulating BC stem/progenitor functions across different organ systems [32, 33].
In summary, we have demonstrated that the INSR/IGF1R mediated signaling pathway regulates the stem/progenitor function of human airway BC and is activated in response to co-culture with lung microvasculature EC. In conjunction with studies demonstrating a critical role for EC in directing morphogenesis, distal stem cell differentiation and regeneration of the murine lung [34–36], our data further supports the concept that EC play a similar role in the human airways and are capable of regulating BC stem/progenitor function. Therefore, cross-talk between BC and EC may play a significant role in maintaining normal airway epithelial structure during normal turnover and repair.
Acknowledgments
We thank N. Mohamed for help in preparing this manuscript. These studies were supported, in part, by R01 HL107882, P20 HL113443. KG was supported, in part by a NYSTEM Fellowship. Research reported in this publication was supported by NIH and the Family Smoking Prevention and Tobacco Control Act. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration.
Footnotes
Conflict of interest: The authors declare no potential conflict of interest.
Role of sponsor/funder: The funders of this study had no role in the design and conduct of the study; not in the collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
References
- 1.Crystal RG, Randell SH, Engelhardt JF, Voynow J, Sunday ME. Airway epithelial cells: current concepts and challenges. Proc Am Thorac Soc. 2008;5(7):772–7. doi: 10.1513/pats.200805-041HR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knight DA, Holgate ST. The airway epithelium: structural and functional properties in health and disease. Respirology. 2003;8(4):432–46. doi: 10.1046/j.1440-1843.2003.00493.x. [DOI] [PubMed] [Google Scholar]
- 3.Tam A, Wadsworth S, Dorscheid D, Man SF, Sin DD. The airway epithelium: more than just a structural barrier. Ther Adv Respir Dis. 2011;5(4):255–73. doi: 10.1177/1753465810396539. [DOI] [PubMed] [Google Scholar]
- 4.Tilley AE, Walters MS, Shaykhiev R, Crystal RG. Cilia dysfunction in lung disease. Annu Rev Physiol. 2015;77:379–406. doi: 10.1146/annurev-physiol-021014-071931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans MJ, Van Winkle LS, Fanucchi MV, Plopper CG. Cellular and molecular characteristics of basal cells in airway epithelium. Exp Lung Res. 2001;27(5):401–15. doi: 10.1080/019021401300317125. [DOI] [PubMed] [Google Scholar]
- 6.Hackett NR, Shaykhiev R, Walters MS, Wang R, Zwick RK, Ferris B, et al. The human airway epithelial basal cell transcriptome. PLoS One. 2011;6(5):e18378. doi: 10.1371/journal.pone.0018378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hackett TL, Shaheen F, Johnson A, Wadsworth S, Pechkovsky DV, Jacoby DB, et al. Characterization of side population cells from human airway epithelium. Stem Cells. 2008;26(10):2576–85. doi: 10.1634/stemcells.2008-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hajj R, Baranek T, Le NR, Lesimple P, Puchelle E, Coraux C. Basal cells of the human adult airway surface epithelium retain transit-amplifying cell properties. Stem Cells. 2007;25(1):139–48. doi: 10.1634/stemcells.2006-0288. [DOI] [PubMed] [Google Scholar]
- 9.Hogan BL, Barkauskas CE, Chapman HA, Epstein JA, Jain R, Hsia CC, et al. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell. 2014;15(2):123–38. doi: 10.1016/j.stem.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA. 2009;106(31):12771–5. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rock JR, Randell SH, Hogan BL. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech. 2010;3(9–10):545–56. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaykhiev R, Zuo WL, Chao I, Fukui T, Witover B, Brekman A, et al. EGF shifts human airway basal cell fate toward a smoking-associated airway epithelial phenotype. Proc Natl Acad Sci USA. 2013;110(29):12102–7. doi: 10.1073/pnas.1303058110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Staudt MR, Buro-Auriemma LJ, Walters MS, Salit J, Vincent T, Shaykhiev R, et al. Airway Basal stem/progenitor cells have diminished capacity to regenerate airway epithelium in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;190(8):955–8. doi: 10.1164/rccm.201406-1167LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warner SM, Hackett TL, Shaheen F, Hallstrand TS, Kicic A, Stick SM, et al. Transcription factor p63 regulates key genes and wound repair in human airway epithelial basal cells. Am J Respir Cell Mol Biol. 2013;49(6):978–88. doi: 10.1165/rcmb.2012-0447OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCulley D, Wienhold M, Sun X. The pulmonary mesenchyme directs lung development. Curr Opin Genet Dev. 2015;32:98–105. doi: 10.1016/j.gde.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ojo O, Lagan AL, Rajendran V, Spanjer A, Chen L, Sohal SS, et al. Pathological changes in the COPD lung mesenchyme--novel lessons learned from in vitro and in vivo studies. Pulm Pharmacol Ther. 2014;29(2):121–8. doi: 10.1016/j.pupt.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Tadokoro T, Wang Y, Barak LS, Bai Y, Randell SH, Hogan BL. IL-6/STAT3 promotes regeneration of airway ciliated cells from basal stem cells. Proc Natl Acad Sci U S A. 2014;111(35):E3641–9. doi: 10.1073/pnas.1409781111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curradi G, Walters MS, Ding BS, Rafii S, Hackett NR, Crystal RG. Airway basal cell vascular endothelial growth factor-mediated cross-talk regulates endothelial cell-dependent growth support of human airway basal cells. Cell Mol Life Sci. 2012;69(13):2217–31. doi: 10.1007/s00018-012-0922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding BS, Gomi K, Rafii S, Crystal RG, Walters MS. Endothelial MMP14 is required for endothelial-dependent growth support of human airway basal cells. J Cell Sci. 2015;128(16):2983–8. doi: 10.1242/jcs.168179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franzdottir SR, Axelsson IT, Arason AJ, Baldursson O, Gudjonsson T, Magnusson MK. Airway branching morphogenesis in three dimensional culture. Respir Res. 2010;11:162. doi: 10.1186/1465-9921-11-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomi K, Arbelaez V, Crystal RG, Walters MS. Activation of NOTCH1 or NOTCH3 signaling skews human airway basal cell differentiation toward a secretory pathway. PLoSOne. 2015;10(2):e0116507. doi: 10.1371/journal.pone.0116507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walters MS, Gomi K, Ashbridge B, Moore MA, Arbelaez V, Heldrich J, et al. Generation of a human airway epithelium derived basal cell line with multipotent differentiation capacity. Respir Res. 2013;14:135. doi: 10.1186/1465-9921-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fidler MJ, Shersher DD, Borgia JA, Bonomi P. Targeting the insulin-like growth factor receptor pathway in lung cancer: problems and pitfalls. Ther Adv Med Oncol. 2012;4(2):51–60. doi: 10.1177/1758834011427576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iams WT, Lovly CM. Molecular Pathways: Clinical Applications and Future Direction of Insulin-like Growth Factor-1 Receptor Pathway Blockade. Clin Cancer Res. 2015;21(19):4270–7. doi: 10.1158/1078-0432.CCR-14-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malaguarnera R, Belfiore A. The emerging role of insulin and insulin-like growth factor signaling in cancer stem cells. Front Endocrinol (Lausanne) 2014;5:10. doi: 10.3389/fendo.2014.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh P, Alex JM, Bast F. Insulin receptor (IR) and insulin-like growth factor receptor 1 (IGF-1R) signaling systems: novel treatment strategies for cancer. Med Oncol. 2014;31(1):805. doi: 10.1007/s12032-013-0805-3. [DOI] [PubMed] [Google Scholar]
- 27.Tahimic CG, Long RK, Kubota T, Sun MY, Elalieh H, Fong C, et al. Regulation of Ligand and Shear Stress-induced Insulin-like Growth Factor 1 (IGF1) Signaling by the Integrin Pathway. J Biol Chem. 2016;291(15):8140–9. doi: 10.1074/jbc.M115.693598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moreno-Barriuso N, Lopez-Malpartida AV, de Pablo F, Pichel JG. Alterations in alveolar epithelium differentiation and vasculogenesis in lungs of LIF/IGF-I double deficient embryos. Dev Dyn. 2006;235(8):2040–50. doi: 10.1002/dvdy.20842. [DOI] [PubMed] [Google Scholar]
- 29.Pais RS, Moreno-Barriuso N, Hernandez-Porras I, Lopez IP, De Las Rivas J, Pichel JG. Transcriptome analysis in prenatal IGF1-deficient mice identifies molecular pathways and target genes involved in distal lung differentiation. PLoSOne. 2013;8(12):e83028. doi: 10.1371/journal.pone.0083028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pichel JG, Fernandez-Moreno C, Vicario-Abejon C, Testillano PS, Patterson PH, de Pablo F. Developmental cooperation of leukemia inhibitory factor and insulin-like growth factor I in mice is tissue-specific and essential for lung maturation involving the transcription factors Sp3 and TTF-1. Mech Dev. 2003;120(3):349–61. doi: 10.1016/s0925-4773(02)00449-5. [DOI] [PubMed] [Google Scholar]
- 31.Galvis LA, Holik AZ, Short KM, Pasquet J, Lun AT, Blewitt ME, et al. Repression of Igf1 expression by Ezh2 prevents basal cell differentiation in the developing lung. Development. 2015;142(8):1458–69. doi: 10.1242/dev.122077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gunschmann C, Stachelscheid H, Akyuz MD, Schmitz A, Missero C, Bruning JC, et al. Insulin/IGF-1 controls epidermal morphogenesis via regulation of FoxO-mediated p63 inhibition. Dev Cell. 2013;26(2):176–87. doi: 10.1016/j.devcel.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stachelscheid H, Ibrahim H, Koch L, Schmitz A, Tscharntke M, Wunderlich FT, et al. Epidermal insulin/IGF-1 signalling control interfollicular morphogenesis and proliferative potential through Rac activation. EMBO J. 2008;27(15):2091–101. doi: 10.1038/emboj.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding BS, Nolan DJ, Guo P, Babazadeh AO, Cao Z, Rosenwaks Z, et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell. 2011;147(3):539–53. doi: 10.1016/j.cell.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JH, Bhang DH, Beede A, Huang TL, Stripp BR, Bloch KD, et al. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell. 2014;156(3):440–55. doi: 10.1016/j.cell.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramasamy SK, Kusumbe AP, Adams RH. Regulation of tissue morphogenesis by endothelial cell-derived signals. Trends Cell Biol. 2015;25(3):148–57. doi: 10.1016/j.tcb.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]