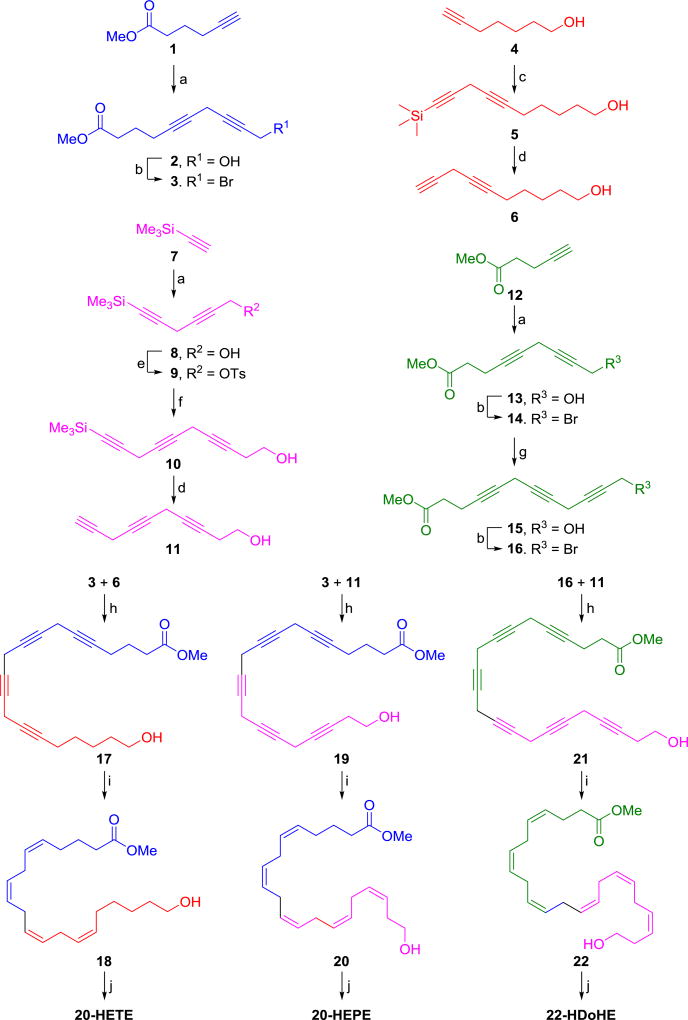
Scheme 1.
Synthetic routes of compounds 20-HETE, 20-HEPE, and 22-HDoHE.
Reagents and conditions: (a) CuI, NaI, Cs2CO3, 4-chloro-2-butyn-1-ol, DMF, rt, 12h; (b) PPh3, CBr4, DCM, 0 °C, 2h; (c) CuI, NaI, Cs2CO3, 3-bromo-1-(trimethylsilyl)-1-propyne, DMF, rt, 12h; (d) 1M TBAF in THF, AcOH, THF, rt, 12h; (e) Et3N, DMAP, p-TsCl, DCM, 0 °C to rt, 12h; (f) CuI, NaI, Cs2CO3, 3-butyn-1-OH, DMF, rt, 12h; (g) CuI, NaI, Cs2CO3, propargyl alcohol, DMF, rt, 12h; (h) CuI, NaI, Cs2CO3, DMF, rt, 12h; (i) Lindlar catalyst, 2-methyl-2-butene:MeOH:pyridine (4:4:1), H2, rt, 18h-2d (note: 1:1 MeOH/EtOAc was used instead of MeOH for both 20 and 22); (j) 1N NaOH, MeOH, rt, 5h.