Figure 1.
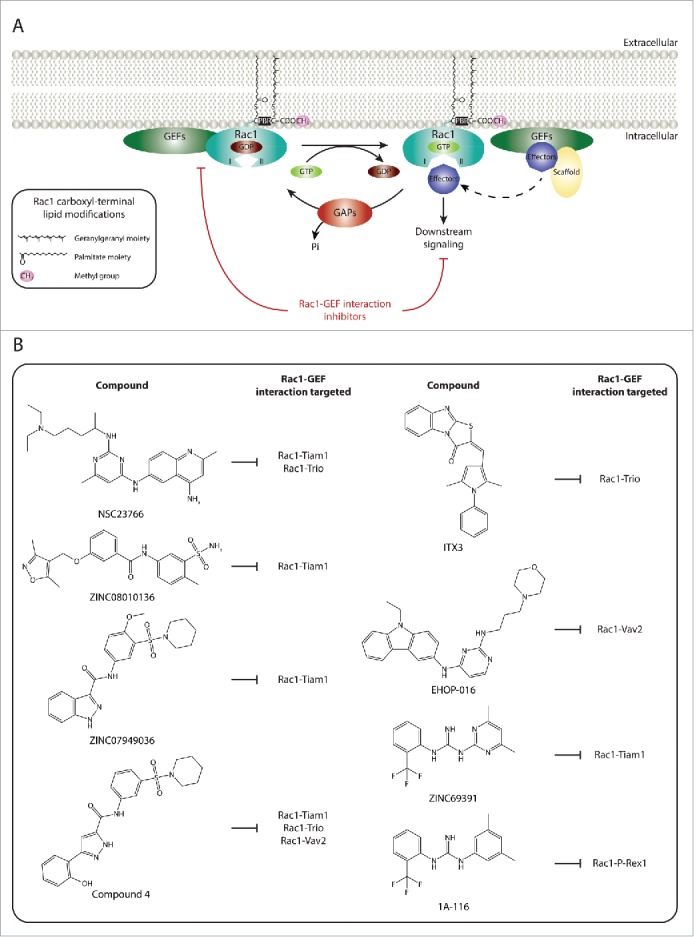
Targeting Rac1 activation and downstream signaling via blocking Rac1-GEF interactions. (A) Similarly to other Rho GTPases, Rac1 cycles between an inactive guanosine diphosphate (GDP)-bound state and an active guanosine triphosphate (GTP)-bound state. This cycle is regulated, in part, by guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs). Following Rac1 prenylation, in which a geranylgeranyl moiety is covalently attached to cysteine 189, carboxyl-terminal methylation and the addition of a palmitate moiety on cysteine 178, GDP-bound Rac1 associates with the plasma membrane. The Polybasic region (PBR) of Rac1 has also been implicated in targeting Rac1 to the plasma membrane. This, in turn, allows GEFs present at the plasma membrane to bind to Rac1 and facilitate the exchange of GDP for GTP, thereby activating Rac1. As a result of GTP binding, Rac1 undergoes a conformational change in its switch I and switch II regions (depicted as I and II, respectively) that promotes binding with downstream effectors, thus translating upstream signals into downstream responses. In addition to Rac1 activation, GEFs can also serve as scaffolding proteins, via indirectly or directly associating to Rac1 effectors, thereby enriching specific Rac1-effector complexes and dictating Rac1 downstream signaling cascades. It is unclear, however, whether plasma membrane localization is essential for the GEF scaffolding function. In contrast, GAPs serve as Rac1 inhibitors, through enhancing the intrinsic GTPase activity of Rac1 and promoting the hydrolysis of bound GTP. (B) Given the importance of GEFs in activating Rac1 and mediating Rac1 downstream signaling, a number of Rac1 specific inhibitors have been developed that target Rac1 activation via blocking Rac1-GEF interactions. Examples of chemical structures and selectivity toward specific Rac1-GEF interactions are outlined.
