Figure 1.
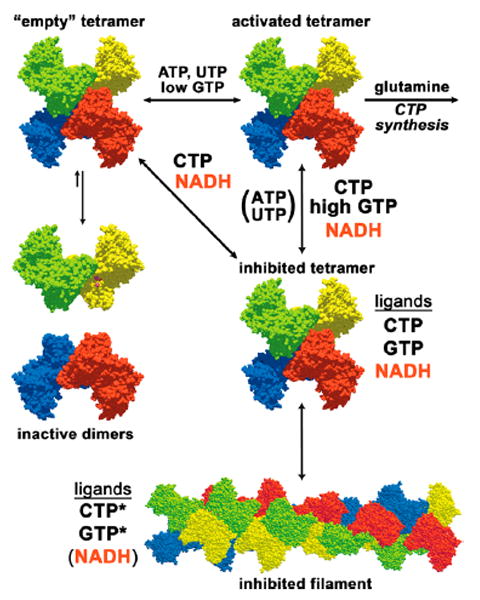
Nucleotide regulation of EcCTPS. EcCTPS is in a dimer–tetramer equilibrium favoring the tetramer in the presence of nucleotides ATP and UTP, since their binding sites are completed upon tetramerization (see refs 2 and 23). GTP (<250 μM) activates glutamine hydrolysis. Product feedback inhibitor CTP binds the tetramer, competitively preventing UTP binding. CTP-bound tetramers are also in equilibrium with an inhibited filament (see ref 31). It is unknown whether EcCTPS dimers bind GTP. Mutual enhancements of each other’s inhibitory potencies by CTP, NADH, and GTP and the lack of competition of GTP and NADH with ATP and UTP substrates suggest that they preferentially bind an inhibited tetramer conformation distinct from the “active” one. CTP and NADH can bind this conformation in the absence of other ligands. Responses to a filament-disrupting mutation suggest that CTP and GTP also preferentially interact with the filament (indicated by an asterisk) while NADH does not.
