Figure 6.
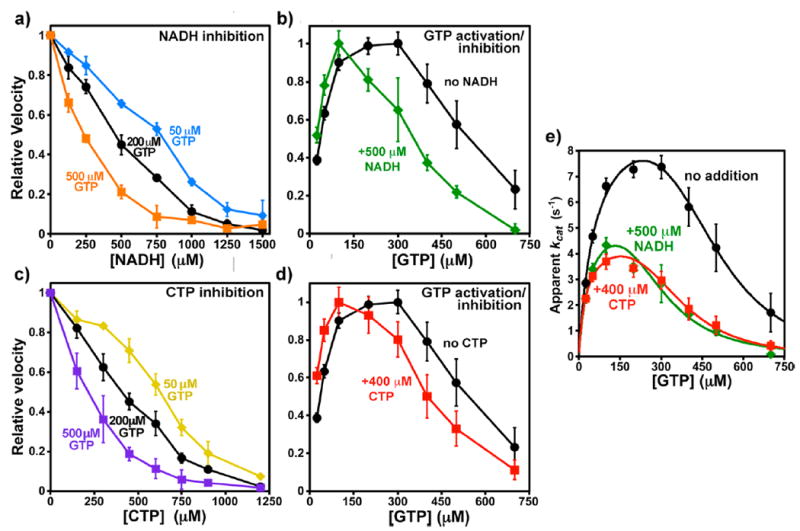
Interactions of CTP or NADH with GTP. Apparent EC50 and IC50 values are given in Table 3. (a) Relative CTP synthesis velocities with 0–1500 μM NADH in the presence of GTP at 50 μM (blue diamonds), 200 μM (black circles), and 500 μM (orange squares). Error bars indicate the standard deviation for three experiments after normalizing to V0 equal to one. The V0 values were 0.64 ± 0.01, 1.21 ± 0.05, and 0.70 ± 0.04 μM s−1, respectively. (b) Relative velocities as a function of 25–700 μM GTP in the absence (black circles) or presence (green squares) of 500 μM NADH. Error bars indicate the standard deviation for four experiments after averaging and then normalizing to Vmax equal to one. The Vmax values were 1.47 ± 0.09 and 0.86 ± 0.06 μM s−1, respectively. (c) Relative velocities with 0–1200 μM CTP in the presence of GTP at 50 μM (yellow diamonds), 200 μM (black circles), and 500 μM (purple squares). Error bars indicate the standard deviation for three experiments after normalizing to V0 equal to one. The V0 values were 0.65 ± 0.01, 1.16 ± 0.10, and 0.77 ± 0.09 μM s−1, respectively. (d) Relative velocities as a function of 25–700 μM GTP in the absence (black circles) or presence (green squares) of 500 μM NADH. Error bars indicate the standard deviation for four experiments after averaging and then normalizing to Vmax equal to one. The Vmax values were 1.47 ± 0.09 and 0.74 ± 0.06 μM s−1, respectively. (e) Fit of GTP titration data to a two-site inhibition model 10 (see Materials and Methods). Unnormalized kcat values with data from experiments with no inhibitor (black circles), 500 μM NADH (green diamonds), or 400 μM CTP (red squares) are shown (see Table 3), along with curves, calculated from the following parameters fit to the averaged data: (i) uninhibited (black circles), kact = 10.0 s−1, KA = 57 μM, Ki = 301 μM, and ninh = 4.8 (R2 = 0.993); (ii) NADH inhibited (green diamonds) kact = 6.0 s−1, KA = 39 μM, Ki = 175 μM, and ninh = 4.1 (R2 = 0.966); (iii) CTP inhibited (red squares) kact = 4.8 s−1, KA = 27 μM, Ki = 202 μM, and ninh = 4.5 (R2 = 0.962).
