Figure 7.
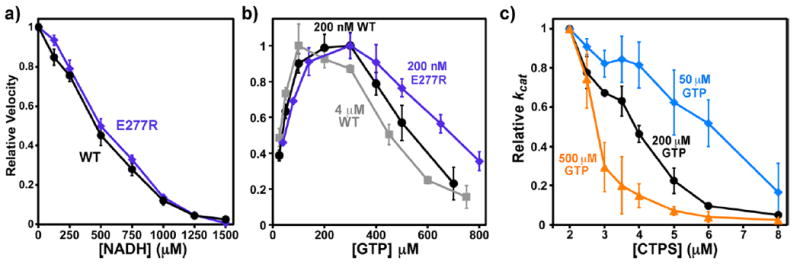
The role of inhibitory filament formation in NADH and GTP inhibition. (a) Relative CTP synthesis velocities of 200 nM WT (black circles) and E277R (see ref 31, in digo diamonds) in the presence of 0–1500 μM NADH normalizing the V0 equal to one (V0 (WT) = 1.24 ± 0.05 μM s−1, V0 (E277R) = 1.07 ± 0.03 μM s−1). Error bars indicate the standard deviation from the average values of three experiments after normalizing V0 equal to one. The filament blocking mutation E277R does not significantly affect NADH inhibition. (b) Relative velocities of 200 nM WT (black circles), 4 μM WT (gray squares), or 200 nM E277R (indigo diamonds) as a function of 25–800 μM GTP. Error bars indicate the standard deviation from the average of four experiments. For comparison purposes, the maximum velocities of each experiment were normalized to one prior, to averaging. The average Vmax values were 1.57 ± 0.11 s−1 (200 nM WT), 14.0 ± 1.7 s−1 (4 μM WT), and 1.60 ± 0.11 μM s−1 (200 nM 277R). The E277R mutation reduced both the activating and inhibitory effects of GTP, while high protein concentrations increased them. The EC50 and IC50 values, respectively, were 38 ± 3 and 540 ± 60 μM (200 nM WT), 27 ± 7 and 455 ± 46 μM (4 μM WT), and 46 ± 6 and 697 ± 4 μM (200 nM E277R). Fitting the data to a two-site activation/inhibition model (fits not shown) yielded the following parameters: for 200 nM WT, kact = 10.7 s−1, KA = 58 μM, Ki = 301 μM, and ninh = 4.7 (R2 = 0.993); for 4 μM WT, kact = 4.7 s−1, KA = 41 μM, Ki = 221 μM, and ninh = 4.1 (R2 = 0.984); and 200 nM E277R, kact = 10.7 s−1, KA = 90 μM, Ki = 342 μM, and ninh = 3.8 (R2 = 0.996). (c) Relative kcat values from at 2–8 μM final EcCTPS in the presence of 50 μM (blue diamonds), 200 μM (black circles), and 500 μM (orange triangles) GTP. Reactions were performed as previously described 31 with annealing carried out at 40 μM EcCTPS. Error bars indicate the standard deviation from the average kcat of three experiments, after normalizing the maximum kcat of each to one. GTP enhances protein-dependent inhibition. The apparent kcat values at 2 μM EcCTPS are 2.9 s−1(50 μM), 5.0 ± 0.1 s−1(200 μM), and 2.7 ± 1.0 s−1(500 μM). The enzyme concentrations for 50% autoinhibition are 6.1 μM (50 μM), 3.9 μM (200 μM), and 2.8 μM (500 μM).
