Abstract
Background
Inducing mitochondrial dysfunction has been recently demonstrated to be an alternative therapeutic strategy for cancer treatment. Doxycycline is an antibiotic that has been shown to have anti-cancer activities in various cancers by way of targeting mitochondria. In this work, we examined whether doxycycline can be repurposed for glioblastoma treatment.
Material/Methods
The effects of doxycycline on the growth, survival, and mitochondrial metabolisms of glioblastoma were investigated. The efficacy of a combination of doxycycline with temozolomide was examined using xenograft mouse model in total number of 40 mice.
Results
Doxycycline targeted glioblastoma cell lines, regardless of their origin, through inhibiting growth and inducing cell death, accompanied by a significant decrease in proliferating cell nuclear antigen (PCNA) and increase in cleaved caspase-3. In addition, doxycycline significantly sensitized glioblastoma cell response to temozolomide in vitro and in vivo. Mechanistically, doxycycline disrupted mitochondrial functions through decreasing mitochondrial membrane potential and mitochondrial respiration. Inducing mitochondrial dysfunctions by using doxycycline led to energy crisis, oxidative stress, and damage as shown by the decreased levels of ATP and the elevated levels of mitochondrial superoxide, intracellular ROS, 8-OHdG, protein carbonylation, and lipid peroxidation. An antioxidant N-acetyl-L-cysteine (NAC) significantly abolished the anti-proliferative and pro-apoptotic effects of doxycycline, demonstrating that doxycycline acts on glioblastoma via inducing oxidative stress.
Conclusions
In our study, we show that the antibiotic doxycycline is effective in targeting glioblastoma through inducing mitochondrial dysfunctions and oxidative stress. Our work also demonstrated the importance of mitochondrial metabolism in glioblastoma.
MeSH Keywords: Doxycycline, Glioblastoma, Mitochondria, Oxidative Stress
Background
Glioblastoma is the most malignant brain tumor in adults with a one year survival rate less than 40% [1]. Standard treatments for glioblastoma are surgery, followed by a combination of radiation and chemotherapy with temozolomide. However, its clinical management is still challenging and its resistance to therapy is attributed to massive vascularization and molecular heterogeneity [2,3]. Studies on transcript profiling and genomic aberrations have shown that glioblastoma is intra- and inter-tumor heterogeneous [4,5]. Therefore, alternative therapeutic strategies for glioblastoma treatment could be to identify compounds/drugs that target what is common between different subclasses of glioblastoma, such as tumor metabolism.
Doxycycline is a FDA-approved antibiotic used in the treatment of a number of types of bacterial and protozoan infections. It kills bacteria by inhibiting protein synthesis via binding to bacterial ribosomes [6]. However, its anti-cancer activities have been recently demonstrated in various studies. Doxycycline has been shown to suppress the growth of cervical cancer, human breast cancer metastasis, and tumor-sphere formation in a panel of cancer cell lines, including lung cancer and prostate cancer cell lines [7–9]. Besides tumor cells, doxycycline also targets cancer stem cells [10]. The mechanisms of doxycycline’s anti-cancer activities might be due to its ability in altering energy metabolism [8,11]. Nevertheless, the effects of doxycycline in glioblastoma are unknown.
In our study, we investigated the effects of doxycycline alone and in combination with temozolomide on glioblastoma using in vitro culture cell system and an in vivo xenograft mouse model. We showed that doxycycline effectively targets glioblastoma cells and enhances the inhibitory effects of temozolomide. We further demonstrated that the inhibitory effects of doxycycline in glioblastoma were attributed to its induction of mitochondrial dysfunction and oxidative stress.
Material and Methods
Human cell lines and drugs
Human glioblastoma cell lines A172 and U87 were purchased from Sigma. Cells were grown in Dulbecco’s Modified Eagle Medium (DMEM) (Life technologies, USA) supplemented with 10% fetal bovine serum (FBS, Hyclone, UK) and 2 mM L-glutamine (Invitrogen) according to manufacturer’s instructions. Doxycycline, N-Acetyl-L-cysteine (NAC) and temozolomide (Sigma, USA) were dissolved in water and DMSO, respectively.
Analysis of cell proliferation and apoptosis
Cells were treated with doxycycline alone or in combination for 72 hours. Cell proliferation was determined by CellTiter 96R AQueous One Solution Cell Proliferation assay kit (Promega, USA) according to manufacturer’s instructions. Cell apoptosis was determined by labeling apoptotic cells with Annexin V-FITC and 7-AAD (BD Biosciences, USA). The percentage of stained cells was then analyzed using flow cytometry and CXP analysis software (Beckman Coulter, USA).
Western blot analyses
Cells were treated with drug for 24 hours. Cells were then lysed by RIPA buffer (Life Technologies Inc, USA) supplemented with protease inhibitor cocktail (Roche, USA). Total protein concentration was measured using the bicinchoninic acid protein assay kit (Thermo Scientific, USA). Equal amount of total proteins were resolved using denaturing Denaturing sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and were then processed for WB analyses using antibodies against PCNA, caspase 3 and β-actin (Santa Cruz Inc., USA).
Metabolic assays
Cells were treated with drugs for 24 hours prior to performing metabolic assays. Oxygen consumption rate (OCR) was measured using a Seahorse XF24 extracellular flux analyzer (Seahorse Bioscience, USA) according to Seahorse Bioscience protocols [12]. Cells were first equilibrated to the unbuffered medium in a CO2-free incubator and then transferred to the XF24 analyzer. Basal OCR was measured at basal condition and maximal OCR was measured after injection of 0.5 μM oligomycin, 0.5 μM carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP) and 0.25 μM antimycin A. Mitochondrial potential was measured by labeling cells with 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl benzimidazolylcarbocyanine iodide (JC-1, Invitrogen) prior to being read on a flow cytometer (Beckman Coulter, USA). ATP levels were measured by using ATPlite Luminiescent Assay kit (Perkin Elmer, USA).
Measurement of mitochondrial superoxide and intracellular reactive oxygen species (ROS)
Cells were treated with drugs for 24 hours. Reactive oxygen species (ROS) levels were determined by incubating cells with 10 μM CM-H2DCFDA (Life Technologies, USA) and measuring absorbance at excitation/emission (ex/em) of 495/525 nm. Mitochondrial superoxide levels were determined by incubating cells with 5 μM MitoSOX Red (Life Technologies, USA) and measuring absorbance at ex/em of 510/580 nm.
Measurement of oxidative damage
Cells were treated with drugs for 24 hours. Oxidative DNA damage was determined by quantifying 8-hydroxy-2′-deoxyguanosine (8-OHdG) levels using the OxiSelect Oxidative DNA Damage ELISA Kit (Cell Biolabs). Protein carbonylation and lipid peroxidation indicating protein and lipid damage were measured using the Protein Carbonyl ELISA Kit (Enzo LifeSciences) and the Lipid Peroxidation MDA Assay Kit (Abcam, USA) according to the manufacturer’s instructions.
Glioblastoma xenograft in SCID mouse
All procedures were approved by the Institutional Animal Care and Use Committee of Hubei Hospital of Integrated Traditional Chinese and Western Medicine. Twenty SCID mice were randomly placed into four groups. All mice were subcutaneously injected 100 μL 50%/50% PSB/Matrigel (BD Biosciences, USA) containing ten million A172 cells in the flank. After development of palpable tumors, the mice were treated with vehicle, intraperitoneal doxycycline at 100 mg/kg, oral temozolomide at 20 mg/kg or combination of both daily for 30 days. Tumor volume was determined by using the formula: volume length×width2/2.
Statistical analyses
For in vitro cell assays, the error bars indicated the value of standard deviation among three independent experiments. For in vivo mouse model work, the error bars indicated the value of standard deviation among 10 mice in each group. Unpaired Student’s t-test was used in the statistical analysis for a comparison of categorical variables. A p value less than 0.05 was deemed as statistically significant.
Results
Doxycycline effectively targeted glioblastoma cells
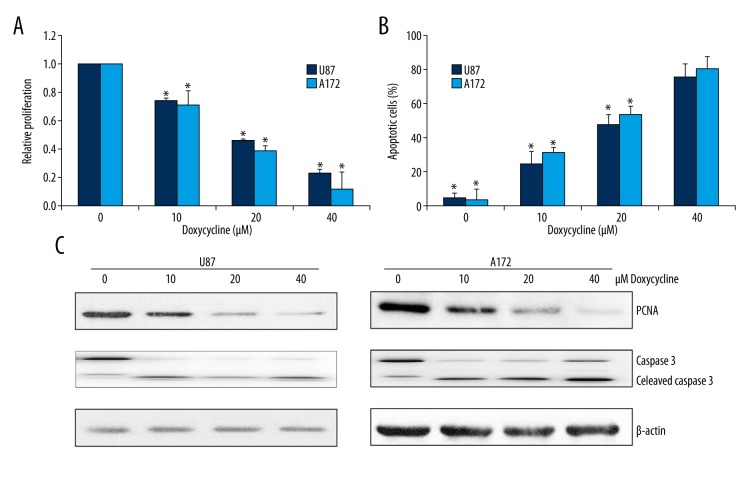
We first investigated whether doxycycline has effects on growth and survival of human glioblastoma using U87 and A172 cell lines. Both cell lines are derived from patients with glioblastoma and have been widely used as glioblastoma cell models [13]. The MTS proliferation results showed that doxycycline at 10 μM to 40 μM significantly inhibited proliferation of U87 and A172 cells in a dose-dependent manner (Figure 1A). Doxycycline also induced apoptosis in glioblastoma cells as assessed by quantifying the percentage of Annexin V and 7-AAD positive cells (Figure 1B). Notably, the effector concentration for half-maximal response (EC50) of doxycycline to A172 and U87 was similar at ~20 μM (Figure 1A, 1B), suggesting that doxycycline effectively targets glioblastoma regardless of their cellular origin. The inhibitory effects of doxycycline on growth and survival of glioblastoma cells were further confirmed by the decreased level of proliferating cell nuclear antigen (PCNA) and increased level of cleaved caspase 3 in A172 and U87 cells exposed to doxycycline (Figure 1C). Doxycycline induced apoptosis in normal primary neuronal cells to a significant lesser extent than in glioblastoma cells (Supplementary Figure 1), suggesting that glioblastoma cells were more sensitive to normal neuronal cells than neuron cancer cells.
Figure 1.
Doxycycline significantly inhibits proliferation and induces apoptosis in glioblastoma cancer cells. Doxycycline inhibits proliferation (A) and induces apoptosis (B) of two glioblastoma cell lines A172 and U87 in a dose-dependent manner. (C) Representative Western blot image showing the levels of PCNA and cleaved caspase 3 in A172 and U87 exposed to doxycycline. These data were derived from three independent experiments. * p<0.05 compared to control.
Doxycycline significantly induced mitochondrial dysfunctions and oxidative stress in glioblastoma cells
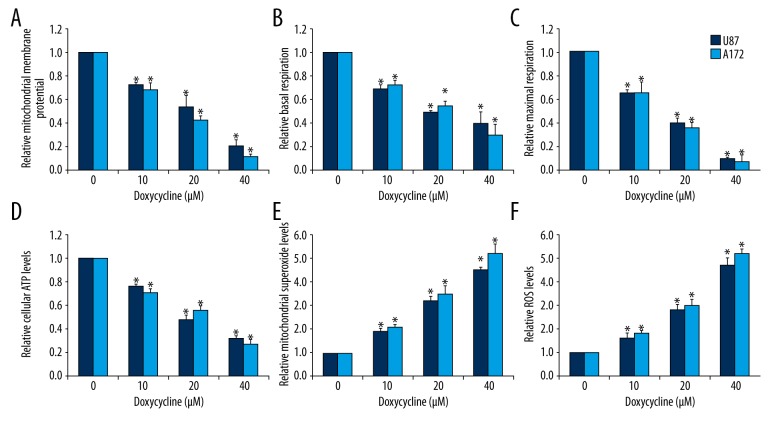
Doxycycline has been reported to target cancer cells via inhibiting mitochondrial biogenesis [8,9]. Given the importance of mitochondrial metabolism in cancer cells, we investigated whether doxycycline affects mitochondrial functions in glioblastoma cells by measuring mitochondrial membrane potential, mitochondrial respiration, and ATP levels. We observed decreased mitochondrial membrane potential in glioblastoma cells treated with doxycycline (Figure 2A). Mitochondrial respiration was measured at both basal and maximal conditions using the Seahorse XF24 extracellular flux analyzer. Doxycycline decreased basal level of mitochondrial respiration as well as respiratory capacity in glioblastoma cells (Figure 2B, 2C). Consistently, ATP levels were decreased in doxycycline-treated U87 and A172 cells (Figure 2D). These results correlated well with each other, demonstrating that doxycycline disrupted mitochondrial functions in glioblastoma cells.
Figure 2.
Doxycycline significantly induces mitochondrial dysfunction and oxidative stress in glioblastoma cells. Doxycycline significantly decreases mitochondrial membrane potential (A), basal (B) and maximal OCR (C), and ATP levels (D) in A172 and U87 cells. Doxycycline significantly increases mitochondrial superoxide (E) and intracellular ROS (F) levels in A172 and U87. These data were derived from three independent experiments. The concentrations of pyrvinium and paclitaxel used in the combination studies were 0.1 μM and 0.5 μM, respectively. * p<0.05 compared to control.
Mitochondrial dysfunction is one of the main sources of oxidative stress in normal and cancer cells [14]. In cell lines with mitochondrial dysfunctions in glioblastoma cells exposed to doxycycline, we also observed increased levels of mitochondrial superoxide (a precursor to many other forms of ROS) and intracellular ROS (Figure 2E, 2F). These results clearly demonstrated that doxycycline induced energy crisis which leads to oxidative stress in glioblastoma cells.
Doxycycline caused oxidative damage to glioblastoma cells
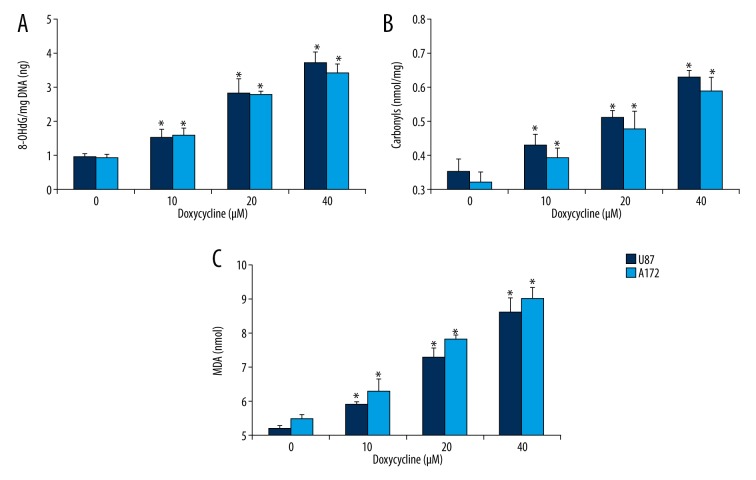
We further investigated whether the oxidative stress induced by doxycycline caused any oxidative damage to glioblastoma cells. 8-hydroxy-2′-deoxyguanosine (8-OHdG, an oxidized DNA by product), protein carbonyls (a modification of proteins resulting from oxidative damage) and MDA (an end product of lipid peroxidation) were quantified. We found significantly elevated levels of 8-OHdG, protein carbonylation, and lipid peroxidation in A172 and U87 cells exposed to doxycycline (Figure 3), indicating that oxidative stress induced by doxycycline caused oxidative damage to glioblastoma cells.
Figure 3.
Doxycycline significantly induces oxidative damage in glioblastoma cells. Doxycycline significantly increases levels of 8-OHdG (A), carbonyls (B) and malondialdehyde (MDA, C) in A172 and U87 cells. * p<0.05 compared to control.
Antioxidants reversed the effects of doxycycline in glioblastoma cells
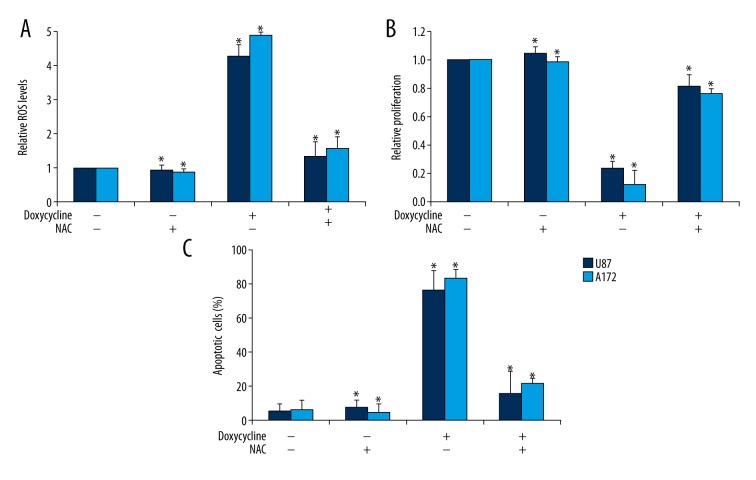
To confirm that doxycycline acts on glioblastoma cells via oxidative damage in lung cancer cells, we investigated whether antioxidants NAC or vitamin C can rescue the effects of doxycycline in glioblastoma cells. NAC is a ROS scavenger that acts through inhibition of thiol redox–mediated ROS production [15]. We found that the effect of doxycycline in inducing oxidative stress was abolished by NAC or vitamin C in U87 and A172 cells (Figure 4A and Supplementary Figure 2A). Importantly, NAC or vitamin C significantly reversed the anti-proliferative and pro-apoptotic effects of doxycycline in U87 and A172 cells (Figure 4B, 4C and Supplementary Figure 2B, 2C), suggesting that oxidative stress was required for the action of doxycycline in glioblastoma cells.
Figure 4.
NAC significantly reveres the effects of doxycycline in glioblastoma cells. The effects of doxycycline in increasing ROS levels (A), inhibiting proliferation (B) and inducing apoptosis (C) in A172 and U87 cells were abolished by NAC. 10 mM NAC and 40 μM doxycycline were added to the medium. * p<0.05 compared to control.
Doxycycline sensitizes glioblastoma cells to chemotherapy in vitro and in vivo
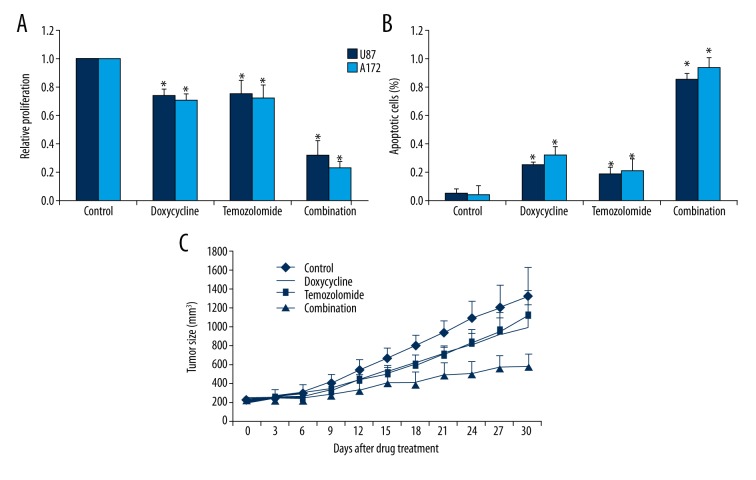
To further explore the translational potential of doxycycline into clinics, we investigated the combinatory effects of doxycycline with standard chemotherapeutic drug and its in vivo efficacy. We found that the combination of doxycycline and temozolomide resulted in greater efficacy than doxycycline or temozolomide alone in inhibiting proliferation and inducing apoptosis in A172 and U87 cells (Figure 5A, 5B). We next established a glioblastoma xenograft mouse model by injecting A172 cells into the flank of SCID mice. After the development of palpable tumors, mice were treated with either vehicle, doxycycline, temozolomide, or a combination for 30 days. All mice tolerated the treatment well. Doxycycline at 100 mg/kg and temozolomide at 20 mg/kg alone slightly inhibited tumor growth (Figure 5C). In contrast, the combination of doxycycline and temozolomide significantly inhibited tumor growth compared to single drug alone throughout the whole treatment duration (Figure 5C).
Figure 5.
Doxycycline significantly enhances the inhibitory effects of temozolomide in glioblastoma in vitro and in vivo. Combination of doxycycline and temozolomide is more superior in inhibiting proliferation (A) and inducing apoptosis (B) than single drug alone in glioblastoma cells. The concentrations of doxycycline and temozolomide used in combination studies were 10 μM and 5 μM, respectively. (C) Combination of doxycycline and temozolomide further inhibits A172 tumor growth in SCID mice compared to single drug alone. 100 mg/kg intraperitoneal doxycycline and 20 mg/kg oral temozolomide or combination of both was given once per day for 30 days. * p<0.05 compared to single arm treatment.
Discussion
Energy metabolism presents a potential therapeutic target for cancer given that tumors are fast growing and highly demanding of energy in comparison to surrounding normal tissues [16–18]. Interestingly, contrary to the classical Warburg view, recent studies have suggested that tumor cells in nutritionally-compromised environments rely on mitochondrial metabolism for energy metabolism and survival [19,20]. Inhibiting mitochondrial functions target not only tumor cells but also quiescent tumor stem cells [8,19–21]. In this work, we report that doxycycline effectively targeted glioblastoma and sensitized glioblastoma cells to chemotherapy through disrupting mitochondrial functions and inducing oxidative stress. Doxycycline is an attractive candidate for glioblastoma treatment as it is already an FDA-approved drug with known pharmacokinetics and toxicity.
We used both an in vitro culturing system and an in vivo xenograft mouse model to demonstrate the efficacy of doxycycline in glioblastoma. The two human glioblastoma cell lines used, A172 and U87, differ in genetic profiling and cellular origin [13]. Doxycycline significantly induced apoptosis and inhibited proliferation of both A172 and U87 cells (Figure 1A, 1B), suggesting that doxycycline was active against glioblastoma regardless of molecular heterogeneity. The anti-proliferative and pro-apoptotic effects of doxycycline were further confirmed by the reduction in PANC and increase in cleaved caspase-3 levels (Figure 1C). Importantly, the combination of doxycycline with temozolomide was more effective in inhibiting proliferation and inducing apoptosis of glioblastoma cells than doxycycline or temozolomide alone (Figure 5A, 5B), suggesting that doxycycline enhances the responsiveness of chemo-resistant glioblastoma cells. This was consistent with the report by Onoda et al. that the combination of doxycycline with cyclooxygenase-2 inhibitor resulted in greater efficacy in colorectal cancer cells compared to single drug alone. In addition, doxycycline’s in vivo efficacy as single drug and as a combination therapy with temozolomide has been demonstrated in a glioblastoma xenograft mouse model (Figure 5C). The inhibitory effects of doxycycline have been demonstrated in cervical cancer and breast cancer [7,9,10]. Our data supports the previous work on the anti-cancer activities of doxycycline and adds glioblastoma to the growing list of doxycycline-targeted cancers.
We next demonstrated that the mechanism of action of doxycycline in glioblastoma was the disruption of mitochondrial functions and induction of oxidative stress. We showed that doxycycline induced mitochondrial dysfunctions via decreasing mitochondrial membrane potential and respiration (Figure 2A–2C), leading to reduction in ATP generation in glioblastoma cells (Figure 2D). This finding was in agreement with previous work that showed doxycycline impaired mitochondrial respiration in cervical cancer cells and altered metabolism in various tumor cell lines and resulted in inhibition of growth and survival in tumor cells [9,11]. Various antibiotics, such as tigecycline and levofloxacin, have been demonstrated to inhibit cancer cells or cancer stem cells via targeting mitochondrial functions [20,22]. The critical role of mitochondrial metabolism has been shown in not only tumor cells but also quiescent tumor stem cells [8,19–21]. Our work together with the previous work suggests that the inhibitory effects of some antibiotics on mitochondrial functions can be used to eradicate cancer cells, including cancer stem cells.
We further showed that doxycycline significantly increases the levels of mitochondrial superoxide and intracellular ROS in glioblastoma cells (Figure 2E, 2F). These results suggested that doxycycline caused oxidative stress. We further showed that doxycycline increased the levels of 8-OHdG, protein carbonylation, and lipid peroxidation (Figure 3), suggesting that oxidative stress induced by doxycycline caused oxidative damage in cellular DNA, protein, and lipids in glioblastoma cells. Although mitochondria has been identified as a target of doxycycline in various types of tumor cells [9,11], we are the first to demonstrate that oxidative stress and damage is the consequence of mitochondrial dysfunctions induced by doxycycline. In addition, antioxidant NAC significantly abolished the effects of doxycycline in inducing oxidative stress, inhibiting proliferation, and inducing apoptosis in glioblastoma cells (Figure 4). These results further confirm that oxidative damage is the mechanism of the action of doxycycline in glioblastoma cells.
Mitochondrial functions and oxidative stress are also involved in various physiological functions of normal cells and biological process [23]. Mitochondrial dysfunction has been reported to induce psychiatric disorders [24]. More research on the understanding of the differential dependence in mitochondrial functions between tumors versus normal cells is needed to develop optimized therapeutic regimens. In addition, the direct molecular target(s) of doxycycline in glioblastoma are not clear although our work showed that mitochondrial dysfunction and oxidative damage were responsible for the action of doxycycline. Identification of doxycycline’s binding partner(s) is important to understand the effectiveness of doxycycline in glioblastoma.
Conclusions
Our work is the first to suggest the potential of doxycycline to be repurposed for glioblastoma treatment, given its efficacy as a single agent and as a combination agent with temozolomide in an in vitro cell culturing system and an in vivo xenograft mouse model. Our work also demonstrated the therapeutic value of inducing mitochondria dysfunction and oxidative stress in the treatment of glioblastoma.
Supplementary Figures
The effects of doxycycline in normal primary neuronal cells. Levofloxacin at 20 and 40 but not 10 μM increases apoptosis in normal neuronal cells. * p<0.5 compared to control.
Vitamin C prevents the effects of doxycycline in inducing oxidative damage, inhibiting proliferation and inducing apoptosis in glioblastoma cells. Doxycycline is ineffective in increasing levels of ROS (A), inhibiting proliferation (B) and inducing apoptosis (C) of U87 and A172 cells in the presence of Vitamin C. * p<0.05, doxycycline alone compared to doxycycline + Vitamin C.
Footnotes
Source of support: This work was supported by a research grant provided by National Natural Science Funds of China (Grant No. 81603095)
Conflict of interest
All authors declare no conflict of interest.
References
- 1.Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neurooncology. 2012;14(Suppl 5):v1–49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerstner ER, Batchelor TT. Antiangiogenic therapy for glioblastoma. Cancer J. 2012;18(1):45–50. doi: 10.1097/PPO.0b013e3182431c6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel AP, Tirosh I, Trombetta JJ, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344(6190):1396–401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brennan CW, Verhaak RG, McKenna A, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–77. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–73. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 6.Chopra I, Hawkey PM, Hinton M. Tetracyclines, molecular and clinical aspects. J Antimicrob Chemother. 1992;29(3):245–77. doi: 10.1093/jac/29.3.245. [DOI] [PubMed] [Google Scholar]
- 7.Duivenvoorden WC, Popovic SV, Lhotak S, et al. Doxycycline decreases tumor burden in a bone metastasis model of human breast cancer. Cancer Res. 2002;62(6):1588–91. [PubMed] [Google Scholar]
- 8.Lamb R, Ozsvari B, Lisanti CL, et al. Antibiotics that target mitochondria effectively eradicate cancer stem cells, across multiple tumor types: Treating cancer like an infectious disease. Oncotarget. 2015;6(7):4569–84. doi: 10.18632/oncotarget.3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Y, Wang X, Li L, Li C. Doxycycline inhibits proliferation and induces apoptosis of both human papillomavirus positive and negative cervical cancer cell lines. Can J Physiol Pharmacol. 2016;94(5):526–33. doi: 10.1139/cjpp-2015-0481. [DOI] [PubMed] [Google Scholar]
- 10.Yang B, Lu Y, Zhang A, et al. Doxycycline induces apoptosis and inhibits proliferation and invasion of human cervical carcinoma stem cells. PLoS One. 2015;10(6):e0129138. doi: 10.1371/journal.pone.0129138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahler E, Sullivan WJ, Cass A, et al. Doxycycline alters metabolism and proliferation of human cell lines. PLoS One. 2013;8(5):e64561. doi: 10.1371/journal.pone.0064561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varum S, Rodrigues AS, Moura MB, et al. Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS One. 2011;6(6):e20914. doi: 10.1371/journal.pone.0020914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie Y, Bergstrom T, Jiang Y, et al. The human glioblastoma cell culture resource: Validated cell models representing all molecular subtypes. EBioMedicine. 2015;2(10):1351–63. doi: 10.1016/j.ebiom.2015.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kudryavtseva AV, Krasnov GS, Dmitriev AA, et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget. 2016;7(29):44879–905. doi: 10.18632/oncotarget.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerksick C, Willoughby D. The antioxidant role of glutathione and N-acetyl-cysteine supplements and exercise-induced oxidative stress. J Int Soc Sports Nutr. 2005;2:38–44. doi: 10.1186/1550-2783-2-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moreno-Sanchez R, Rodriguez-Enriquez S, Marin-Hernandez A, Saavedra E. Energy metabolism in tumor cells. FEBS J. 2007;274(6):1393–418. doi: 10.1111/j.1742-4658.2007.05686.x. [DOI] [PubMed] [Google Scholar]
- 17.Pedersen PL. Tumor mitochondria and the bioenergetics of cancer cells. Prog Exp Tumor Res. 1978;22:190–274. doi: 10.1159/000401202. [DOI] [PubMed] [Google Scholar]
- 18.Zu XL, Guppy M. Cancer metabolism: Facts, fantasy, and fiction. Biochem Biophys Res Commun. 2004;313(3):459–65. doi: 10.1016/j.bbrc.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, de Milito A, Olofsson MH, et al. Targeting mitochondrial function to treat quiescent tumor cells in solid tumors. Int J Mol Sci. 2015;16(11):27313–26. doi: 10.3390/ijms161126020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skrtic M, Sriskanthadevan S, Jhas B, et al. Inhibition of mitochondrial translation as a therapeutic strategy for human acute myeloid leukemia. Cancer Cell. 2011;20(5):674–88. doi: 10.1016/j.ccr.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wen S, Zhu D, Huang P. Targeting cancer cell mitochondria as a therapeutic approach. Future Med Chem. 2013;5(1):53–67. doi: 10.4155/fmc.12.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song M, Wu H, Wu S, et al. Antibiotic drug levofloxacin inhibits proliferation and induces apoptosis of lung cancer cells through inducing mitochondrial dysfunction and oxidative damage. Biomed Pharmacother. 2016;84:1137–43. doi: 10.1016/j.biopha.2016.10.034. [DOI] [PubMed] [Google Scholar]
- 23.Miyazaki T, Yamasaki N, Tsuchiya T, et al. Infectious episodes lead to the oxidative stress response after lung transplantation. Am J Case Rep. 2015;16:255–58. doi: 10.12659/AJCR.893026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stefano GB, Samuel J, Kream RM. Antibiotics may trigger mitochondrial dysfunction inducing psychiatric disorders. Med Sci Monit. 2017;23:101–6. doi: 10.12659/MSM.899478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The effects of doxycycline in normal primary neuronal cells. Levofloxacin at 20 and 40 but not 10 μM increases apoptosis in normal neuronal cells. * p<0.5 compared to control.
Vitamin C prevents the effects of doxycycline in inducing oxidative damage, inhibiting proliferation and inducing apoptosis in glioblastoma cells. Doxycycline is ineffective in increasing levels of ROS (A), inhibiting proliferation (B) and inducing apoptosis (C) of U87 and A172 cells in the presence of Vitamin C. * p<0.05, doxycycline alone compared to doxycycline + Vitamin C.