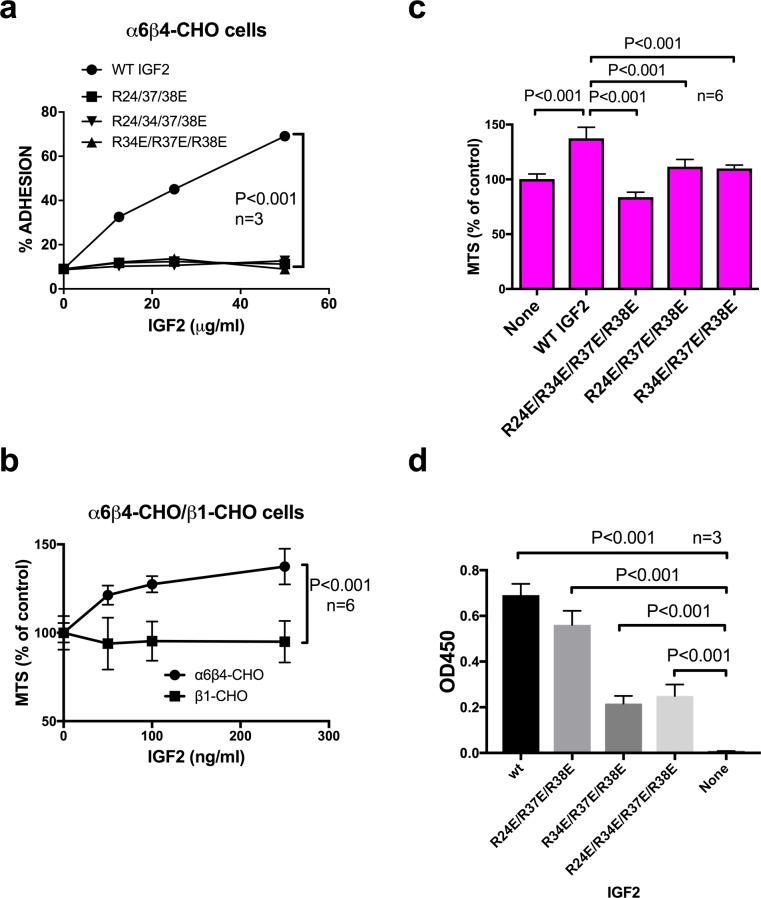
Fig 4. IGF2 binds to α6β4 and induces proliferation of α6β4-CHO cells while integrin-binding defective IGF2 mutants are defective in these functions.
a, α6β4-CHO cells bind to WT IGF2 in adhesion assay. Wells of 96-well microtiter plates were coated with WT IGF2. α6β4-CHO cells were incubated in Tyrode-HEPES buffer containing 1 mM Mg2+, and bound cells were measured. The data are shown as means +/- SEM of triplicate experiments. b, WT IGF2 enhanced proliferation of α6β4-cells but not β1-CHO cells. α6β4-CHO and β1-CHO cells were incubated for 48 hrs with increasing concentrations of WT IGF2 in polyHEMA-coated plates. The data are shown as means +/- SEM (n = 6). c, Integrin-binding defective IGF2 mutants were defective in inducing proliferation of α6β4-CHO cells in MTS assays while WT IGF2 induced it. α6β4-CHO cells (2 x 104 cells/well) were incubated for 48 hrs with WT or mutant IGF2 (250 ng/ml) in polyHEMA-coated plates. The data are shown as means +/- SEM (n = 6). d, Binding of IGF2 mutants to the immobilized IGF1R ectodomain. Wells of 96-well microtiter plate were coated with recombinant human soluble IGF1R at 1 μg/ml in PBS for 1 h at 37°C, and the remaining protein-binding sites were block by incubating with 1 mg/ml BSA for 1 h at room temperature. WT and mutant IGF2 (2.5 μg/50 μl in PBS) were added to the wells and incubated in PBS/0.05% Tween 20 at room temperature for 1 hr. After washing with PBS/0.05% Tween 20, wells were incubated with anti-5His antibody conjugated with HRP, then peroxidase substrate. The data are shown as means +/- SEM (n = 3)