Abstract
Background
Snail intermediate host control is a widely canvassed strategy for schistosomiasis control in endemic countries. While there have been increasing studies on the search for potent molluscicides in the past years, the use of nanoparticulate agents as molluscicides is yet to gain wide attention. The aim of this study was to assess the molluscicidal potential of curcumin-nisin poly lactic acid (PLA) entrapped nanoparticle (CurNisNp) against Biomphalaria pfeifferi, a snail intermediate host for Schistosoma mansoni.
Methodology/Principal findings
CurNisNp formulated by double emulsion method was tested against the young adults, < 1 week, 1-2-week old juveniles, 1 day (blastula) and 7 day-old (hippo-stage) egg masses of B. pfeifferi. Mortality in the different stages was determined after 96-h of exposure at varying concentrations (350, 175, 87.5, 43.75 and 21.88 ppm). The sub-lethal effects of CurNisNp on the hatchability of the 7-day-old egg masses and egg laying capacity of the young adult snails were determined. The CurNisNp diameter, polydispersity index (PDI), zeta potential and drug entrapment efficiency were 284.0 ± 17.9 nm, 0.166 ± 0.03, -16.6 ± 2.45 mV and 35.0% respectively. The < 1 week old juveniles and the 1-day-old egg stage (blastula) of B. pfeifferi with LC50 277.9 ppm and 4279.5 ppm were the most susceptible and resistant stages to the drug respectively. CurNisNp was also observed to cause significant reductions (P<0.05) in egg hatchability and egg laying capacity with strong negative correlation between egg laying capacity and concentration (r = -0.928; P<0.05).
Conclusion/Significance
This study showed that CurNisNp has molluscicidal activities on different developmental stages of B. pfeifferi. It is therefore recommended that the formulation be more optimised to give a nanoparticle with a narrow range monodispersed PDI for better drug distribution and eventual greater molluscicidal activities.
Author summary
Elimination of snail intermediate host of schistosomiasis has been widely advocated as an arm of integrated control for schistosomiasis. This becomes important as reports now abound on development of praziquantel resistance in schistosomes. Nanotechnology has been applied in control of tropical diseases including schistosomiasis. The few available nanomedicines were targeted against the Schistosoma worms without research efforts on the use of the same technology against the molluscan host of the parasite. This study seeks to assess the molluscicidal potential of CurNisNp on Biomphalaria pfeifferi; a snail intermediate host of Schistosoma mansoni. The formulation was tested on the different developmental stages of the snail. The formulation showed decreasing molluscicidal activity with increase in age of the snail. Impairment of the egg laying capacity of the adult snails was related to nanoparticle concentration. The egg was the most resistant stage. It is evident from the study that the formulated nanoparticle had molluscicidal properties and to further harness this potential, optimisation of the formulation to give a narrow range monodispersed polydispersity index for better drug distribution is recommended.
Introduction
Trematodes, the causal agents of schistosomiasis and fascioliasis are important parasites of economic and public health implications in most of sub-Saharan Africa. Schistosomiasis affects over 240 million people worldwide, with up to 700 million individuals living at risk of infection [1]. The disease caused up to 250,000 deaths per year in the last decade [2]. Chemotherapy has been the most adopted means of control of schistosomiasis in developing countries. The increase in number of individuals that need to be treated with praziquantel (PZQ) necessitates the corresponding increase in PZQ deployment in sub-Saharan Africa thus raising concerns about the emergence and establishment of Schistosoma resistance to PZQ [3].
The control of snail intermediate hosts in an attempt to break the parasite transmission cycle is a widely advocated strategy in schistosomiasis control. Niclosamide, a chemical molluscicide has recorded success in this regard, but its toxicity against non-targeted organisms has been a major set-back to its general adoption [4].
Intensive studies have been conducted in search of agents which are more environmentally friendly to combat the intermediate hosts of Schistosoma. Efforts are directed particularly towards molluscicides of plant origin with several studies reported across the world [5–11]. Nevertheless, there is currently no licensed plant-derived molluscicide despite this myriad of studies. This could be due to inability to standardize these findings for wide scale use. The feasibility of plant-induced toxicity on non-targeted organisms cannot also be ruled out.
Nanotechnology has gained increasing interest in biomedicine with the utmost aim of effective delivery of bioactive agents. Particularly it has been widely applied to combat parasitic agents [12,13]. While attention is often drawn towards Schistosoma parasites [14,15], little is known about the application of nanomedicine against the snail intermediate hosts of the parasites. Curcumin and nisin are naturally derived non-toxic compounds [16,17] with a wide range of activities [18–21]. The two compounds have been shown to have antibacterial, anti-inflammatory and anticancer properties [18,19]. Curcumin has been reported to be efficacious against adult Schistosoma mansoni [20,21]. The higher bioavailability of these compounds in nanoparticulate forms and improved efficacy against some biological agents [22,23] could make their combination into a nanoparticulate formulation a desirable molluscicidal agent. The aim of this study was therefore to evaluate the molluscicidal potential of curcumin-nisin PLA entrapped nanoparticle on Biomphalaria pfeifferi, a snail intermediate host of intestinal schistosomes.
Materials and methods
Ethics statement
The institutional animal care and use committee in our Nigerian institutes granted waiver since freshwater snails are not among the selected animals that approval is needed. Also, in Nigeria there are no agencies that issue permit for collection of wild freshwater snails. So, no permit was obtained.
Drug
The test substance (drug): curcumin-nisin poly-lactic acid nanoparticles (CurNisNp), is a yellow biodegradable hygroscopic powder of 35.0% composition by mass of the active ingredients. It was prepared by the double emulsion-diffusion-evaporation method at the National Institute of Immunology, New Delhi, India.
Preparation of curcumin-nisin poly-lactic acid nanoparticle (CurNisNp)
The formulation was prepared by the double emulsion-diffusion-evaporation method. Curcumin and nisin of equal amount (5 mg) was subjected to dissolution in 200 μL 1% polyvinyl alcohol (PVA). The mixture was dispensed into 50 mg of poly lactic acid containing organic solvents and was sonicated for 1 min to obtain a primary emulsion. The emulsion was added dropwise to 16 mL 2% PVA containing 1% sucrose. The secondary emulsion formed was sonicated at 30 W, 40% duty cycle for 3 mins to form a nanosuspension. This was continuously stirred until all the solvents were evaporated. The nanosuspension was subjected to ultracentrifugation (16,000 rpm for 15 min) and then washed. The washing was repeated two times and the formulation was lyophilized with 5% mannitol as cryoprotectant.
Characterization and drug entrapment efficiency of CurNisNp
The physical properties of the formulation including the size, zeta potential and polydispersity index (PDI) were measured by dynamic light scattering method using Zetasizer Nano-ZS (Malvern Instruments, UK). The size and PDI of the nanoparticle were determined by dispersing a homogenous solution of the formulation in sizing cuvette and then measured by Zetasizer Nano-ZS. Clear zeta cell was used for zeta potential analysis. Drug encapsulation efficiency was determined by a modified method described by Dauda et al. [13].
In vitro release kinetics of drug-entrapped nanoparticle
Ten milligram (10 mg) of Cur-Nis-NP was dissolved in 10 mL PBS (140 mM NaCl, 10 mM phosphate buffer, 3 mM KCl, pH 7.4) [21]. The homogenous solution was incubated in a rotary shaker at 200 g. The sample was centrifuged at 16,000 g for 10 min at specific time after which 1 mL of supernatant was withdrawn and then replaced with 1 mL of fresh PBS [13]. Curcumin and nisin (2.5 mg each) was dissolved in 5 mL methanol to form a stock solution (100 μg/mL). The working standard concentrations (5–70 μg/mL) were prepared from the stock with PBS. The UV-absorbance was measured at 290 nm. The UV-absorbance analysis of supernatant from curcumin-nisin PLA entrapped nanoparticle was carried out at different time intervals. The in vitro drug release from the formulated nanoparticle was estimated from the standard plot obtained from UV-absorbance analysis of free curcumin-nisin.
Snail collection
Adults of Biomphalaria pfeifferi were collected from Odo Ona River (latitude 7°21ʹ-7°22ʹN; longitude 3°50ʹ-3°51ʹE) in Ibadan, Oyo State, Nigeria. They were properly washed in water and transferred into plastic containers with good ventilation. The snails were brought to the Parasitology Research Laboratory of the Department of Zoology, University of Ibadan for further analysis. Snails were collected blinded of their infection status and were later subjected to cercariae screening through exposure to sunlight for 1–2 h in dechlorinated tap water. Only clean snails were used for the study.
Snail culture
Twenty five (25) adult B. pfeifferi were transferred into a culture jar (aquarium) lined with a transparent polythene bag containing dechlorinated tap water. The snails were fed with blanched dried lettuce (Lactuca sativa), and CaCO3 pellets were used as calcium supplements. They were maintained at room temperature (26–29°C) under natural light:dark cycles. The egg masses laid by snails were cut out with a scalpel and transferred into a petri dish containing dechlorinated tap water. Incubation was done as previously described [8,24]. The snails hatched within 6-7-days of incubation, and were subsequently transferred and maintained in a larger container to accommodate their growth.
Molluscicidal bioassay activity test
The molluscicidal bioassay activity tests were carried out on the snail developmental stages (<1 week old juveniles, 1–2 weeks old juveniles, and 5–6 weeks old young adults) in line with the WHO guidelines [25,26]. Ten (n = 10) snails were placed in each test container for all the stages tested except the < 1 week old B. pfeifferi juveniles where number of snails exposed was n = 22. The snails at different developmental stages were placed in 40 mL of varying concentrations (350 ppm, 175 ppm, 87.5 ppm, 43.75 ppm and 21.88 ppm diluted with dechlorinated water) of the nanoparticle formulation and mortality was observed after 96-h exposure. Snails’ avoidance or protective behaviours during exposure were observed. Observation and examination for mortality were done using hand lens or dissecting microscope where necessary. The snails that could move or with an active heart beat (as observed under the microscope) were counted as living and vice versa. The percentage mortality was calculated.
Ovicidal activity and egg hatchability
The ovicidal bioassay activity and egg hatchability tests were carried out on the egg masses of uninfected adult B. pfeifferi using 1 day old blastula stage and 6–7 days old pre-hatched hippo- stage respectively in line with the methods [27,28]. Two to three egg masses (adding up to an average of 26 embryos) were harvested from the snail cultures and placed in each test container containing different concentrations of the test material. The egg masses were observed every 24 h for one week and afterwards biweekly for four weeks at room temperature and normal diurnal lightening. After every 24 h, the snail egg masses were examined under the microscope for viability and then the percentage mortality calculated. At the pre-hatched stage, the snail embryos were observed under the microscope for movement within their gelatinous egg masses. The pre-hatched eggs were further examined for number of embryos hatched. The egg hatchability was calculated as percentage difference relative to the total number of eggs exposed.
Egg laying capacity determination
The egg laying capacity of young adult B. pfeifferi snails was determined by maintaining and monitoring snails’ oviposition to assess their reproductive viability daily for 5 days post exposure to CurNisNp. This was achieved by counting the number of egg masses laid by the different groups of young adult snails exposed [27,29]. The total number of eggs laid by treated and control groups of snails were estimated.
All experiments were performed in duplicate with values expressed as mean ±SD. The negative control groups were placed in dechlorinated water.
Statistical analysis
The data were subjected to SPSS version 21 for windows for analysis. Two-way ANOVA was used to test significant differences in snail mortality in different concentrations. Probit regression graphing was used to determine the LC50 and LC90 of the nanoparticulate formulation. Linear Regression analysis and Pearson’s correlation were applied to determine the relationship between snail mortality/egg hatchability/egg laying capacity (fecundity) and test concentrations. P <0.05 was considered statistically significant.
Results
Properties of nanoparticle
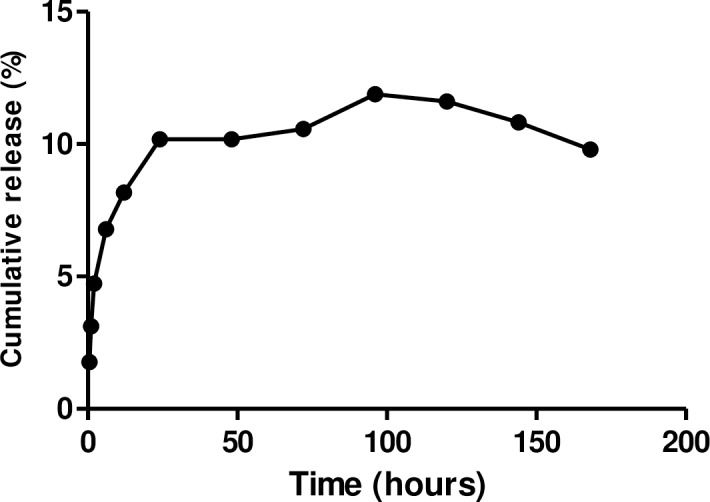
The CurNisNp diameter, PDI, zeta potential and drug entrapment efficiency were 284.0 ± 17.9 nm, 0.166 ± 0.03, -16.6 ± 2.45 mV and 35.0% respectively. The in vitro release of Cur-Nis is presented in Fig 1.
Fig 1. In vitro release of Cur-Nis in formulated nanoparticle.
Snail behaviour and mortality
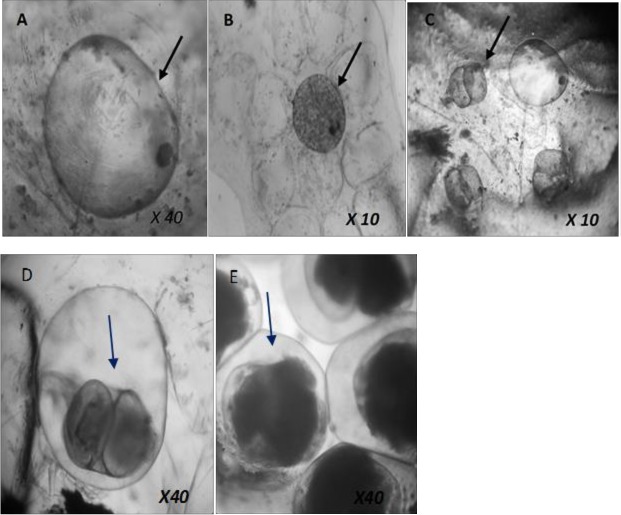
The protective behaviors of the snails following their introduction into the test concentrations included crawling along the walls of the containers, surfacing behavior and partial retraction of their head-foot. Normal crawling activities resumed after only a few minutes. Mortality in snails was not dependent on concentrations of the formulation (P>0.05). However, mortality was significantly higher in the tested concentrations compared with the negative control (P<0.05). The formulation killed more than half of the young adult snails (5–6 week-old) (60.0–70.0%) in concentrations 43.75, 87.5 and 350.0 ppm. The <1 week old juvenile of B. pfeifferi were the most susceptible to CurNisNp with mortality ranging from 82.2–100.0 ppm (Table 1). The 1 day exposed egg masses were the least susceptible group with highest mortality of embryos recorded in 175.0 ppm of CurNisNp. No embryonic death was recorded in 7-day-old egg masses exposed to 175.0 ppm and 43.75 ppm of the nano-formulated drug. Half of the 1-2-week-old juveniles (50.0%) of B. pfeifferi died at 350.0 ppm after 96-h exposure (Table 1). The <1 week-old juvenile snails had the lowest LC50 (277.9 ppm) and LC90 (676.4 ppm) while the 1-day-old egg had the highest LC50 (4279.5 ppm) and LC90 (8184.6 ppm) (Table 2). The photomicrographs of toxicity effects of CurNisNp on B. pfeifferi embryos are presented in Fig 2A–2E (A; dead embryo viewed 1 week after exposure, B; dead embryo viewed 4 weeks after exposure, C; empty shell of dead prehatched stage embryo beside a dead blastula stage embryo viewed 4 weeks after exposure, D; deformed embryo, E; normal embryo at prehatched stage).
Table 1. Percentage mortality (± SD) of freshwater snails at different developmental stages.
| Concentration (ppm) | |||||||
|---|---|---|---|---|---|---|---|
| Age | Stage | 350.0 | 175.0 | 87.5 | 43.75 | 21.88 | Control |
| 1 day | Egg (blastula) | 4.9 ±2.12 | 20.0 ± 2.83 | 3.2 ± 1.41 | 6.5 ±0.00 | 3.2 ± 1.41 | 0.0 |
| 7 days | Egg (hippo) | 25.6 ±7.78 | 0.0 ± 0.0 | 40.5 ± 6.36 | 0.0 ± 0.0 | 47.5 ± 20.50 | 0.0 |
| <1 week | Juvenile | 100.0 ±8.49 | 97.7 ± 2.12 | 88.6 ± 3.54 | 82. 2 ± 2.12 | 93.3 ± 19.80 | 0.0 |
| 1–2 weeks | Juvenile | 50.0 ±1.41 | 45.0 ± 2.12 | 25 ± 0.71 | 20.0 ± 1.41 | 25 ± 0.71 | 0.0 |
| 5–6 weeks | Adult | 70.0 ±0.00 | 20 ± 0.00 | 70 ± 0.00 | 60.0 ± 0.00 | 50 ± 1.41 | 0.0 |
Table 2. Probit analysis of lethal concentrations of CurNisNp against B. pfeifferi at different stages exposed.
| Lethal concentration (ppm) (95% CI) | |||||
|---|---|---|---|---|---|
| Age | Stage | Regression equation | R2 | LC50 | LC90 |
| 1 day | Egg (blastula) | y = 0.0001x + 0.062 | 0.037 | 4279.5 (2645.79–6769.2) | 8184.6 (7476.23–109124.5) |
| 7 days | Egg (hippo) | y = -0.0002x + 0.247 | 0.022 | 1072.7 (872.33–1326.2) | 2767.7 (1927.213–3623.2) |
| <1 week old | Juvenile | y = 0.0004x + 0.867 | 0.557 | 277.9 (210.45–344.5) | 676.4 (510.45–724.6) |
| 1–2 weeks | Juvenile | y = 0.0009x+0.204 | 0.840 | 318.9 (245.12–398.3) | 750.0 (635.89–857.2) |
| 5–6 weeks | Adult | y = 0.0002x + 0.433 | 0.025 | 339.1 (229.7965–448.2) | 2373.4 (1472.34–3258.5) |
Fig 2. Photomicrographs showing dead/deformed and normal B. pfeifferi embryos.
Effects of nanoparticle on snail hatchability
The hatchablity of snails was significantly higher in the negative control than in the exposed groups (P<0.05). The embryos hatching from the gelatinous masses significantly increased with time (P<0.05). No snail was hatched out in the nanoparticulate concentrations 350.0, 175.0 and 87.5 ppm after 24-h exposure. All snails had hatched after 144-h exposure in concentrations 175.0 and 43.75 ppm (Table 3). Snail hatchability was independent of nanoparticle concentration (P>0.05); however, all the hatched snails died within a short period compared with the negative control.
Table 3. Percentage hatchability (± SD) of B. pfeifferi eggs exposed to CurNisNp at the pre-hatched stage.
| Time (hours) | ||||||
|---|---|---|---|---|---|---|
| Conc. (ppm) | 24 | 48 | 72 | 96 | 120 | 144 |
| 350.0 | 0.0 ± 0.0 | 7.0 ± 0.7 | 23.3 ± 1.4 | 46.5 ± 2.8 | 74.4 ± 8.5 | 74.4 ± 8.5 |
| 175.0 | 0.0 ± 0.0 | 2.3 ± 0.7 | 27.3 ± 0.7 | 72.7 ± 2.8 | 93.2 ± 2.1 | 100.0 ± 1.4 |
| 87.5 | 0.0 ± 0.0 | 0.0 ±0.0 | 5.8 ± 2.12 | 23.1 ± 1.41 | 34.6 ± 1.4 | 67.3 ± 0.7 |
| 43.75 | 4.4 ± 1.4 | 8.9 ± 0.0 | 33.3 ± 5.0 | 48.9 ± 5.7 | 64.4 ± 3.5 | 100.0 ± 0.7 |
| 21.88 | 3.4 ± 1.4 | 8.5 ± 3.5 | 13.6 ± 5.7 | 33.9 ± 14.1 | 47.5 ± 19.8 | 50.8 ± 21.2 |
| 0.0 | 89.1 ± 121.4 | 100.0 ± 145.0 | 100.0 ± 126.2 | 100.0 ± 101.6 | 100.0 ± 101.3 | 100.0 ± 83.8 |
Effects of nanoparticles on snail fecundity
The egg laying capacity of the young adult snails exposed to CurNisNp was significantly lower than in the negative control (P<0.05). The fecundity rate was also concentration dependent (P<0.05). The young adult snails exposed to 21.88 ppm had the highest egg laying capacity (48.5 ± 2.91) while those exposed to 350.0 ppm showed the lowest egg laying capacity (14.5 ± 4.23) (Table 4). The Pearson correlation showed significant inverse relationship between CurNisNp concentrations and egg laying capacity of the snails (r = -0.928; P<0.05). The average fecundity rate however showed no significant differences with days of exposure of snails (P>0.05).
Table 4. Egg laying capacity (± SD) of snails exposed to CurNisNp.
| Conc. (ppm) | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Total no. of eggs/group |
|---|---|---|---|---|---|---|
| 350.00 | 0.0 ± 0.0 | 3.0 ± 4.24 | 5.0 ± 7.07 | 1.5 ± 2.12 | 5.0 ± 7.07 | 14.5 ± 4.23 |
| 175.00 | 5.0 ± 7.07 | 3.0 ± 4.24 | 5.0 ± 7.07 | 8.5 ± 2.12 | 4.0 ± 5.66 | 26.0 ± 4.58 |
| 87.50 | 0.0 ± 0.0 | 8.0 ± 2.83 | 7.0 ± 1.41 | 3.5 ± 4.95 | 11.0 ± 1.41 | 29.5 ± 4.48 |
| 43.75 | 7.0 ± 1.41 | 8.0 ± 2.83 | 6.0 ± 0.00 | 12.5 ± 0.71 | 7.5 ± 3.54 | 41.0 ± 2.86 |
| 21.88 | 11.0 ± 1.41 | 6.0 ± 0.00 | 11.0 ± 1.41 | 10.0 ± 5.66 | 10.5 ± 2.12 | 48.5 ± 2.91 |
| 0.0 | 13.5 ±0.71 | 15.0 ± 1.41 | 19.0 ± 1.41 | 23.0 ± 7.07 | 25.0 ± 4.24 | 95.5 ± 5.47 |
Discussion
The curcumin-nisin PLA entrapped polymeric nanoparticle used in this study is novel and could serve as an ideal molluscicide. The PDI of the formulation will facilitate moderate distribution [30] and therefore optimisation of the drug formulation to give a narrow range monodispersed PDI for better drug distribution within the target organism is recommended. This is achievable by varying the concentrations of surfactant, organic and aqueous phase, and drug-polymer ratio [13]. In addition, the origin of the drug, as a formulation from nisin and curcumin, suggests that it will exhibit low to zero toxicity. There is presently no study on toxicity of nisin on non-target organisms, but one study has shown safety of extract from Curcuma longa (the parent plant from which curcumin is obtained) on brine shrimps [31].
The snails’ avoidance behaviors following exposure to the test concentrations of the nanoparticulate drug is an indication of possible molluscicidal effects. These observations are in line with those of many Nigerian workers [8,9,32,33] and workers elsewhere [34–36]. The observed crawling out (distress syndrome) from the test concentrations and aggregation at the water-air interface by the exposed snails was taken as an escape or avoidance behavior which has been described by the aforementioned workers. This behavior which is as a result of response to loss of water balance [37] helps to increase their chances of survival and as a result hinder the action of molluscicides [10].
Susceptibility of B. pfeifferi to the CurNisNp was dependent on snail developmental stages. This kind of developmental stage-dependent variation in susceptibility to a molluscicidal agent has also been observed in a previous study [8]. The lack of association between snail mortality and nanoformulation concentration contradicts the findings of other studies [8,32,38–40]. This was particularly observed in those instances where the nanoparticulate drug showed higher activity at low doses when compared with high doses. This contrast with other studies could have been due to the ability of the formulation to penetrate membrane barriers in the organism to reach the target tissues or organ, even at lower doses. The use of nanoparticle formulations may confer an advantage over the use of unbound drug or plant extracts, as employed in the aforementioned studies, as lower doses of nanoparticles could exert similar efficacy. The lower LCs values recorded in the juveniles indicates their higher susceptibility to CurNisNp, but the relatively higher value recorded for the young adult snails could have been due to increase resistance to the formulation as the snails advanced in age. This is reasonable as tolerance to adverse environmental conditions increases with the age-dependent acquisition of better developed organs e.g. mantle and periostracum. Our investigation did not show the concentration-dependent nature of molluscicidal action on embryos reported in earlier studies [8,41]. However, our study shared similar morphological alterations such as deformation of gastrula with other studies [8,39].
Reduction in snail hatchability in nanoparticle exposed groups suggests an increase in bioavailability which ensured delivery of PLA encapsulated drug to the targeted pre-hatched snails within the protective gelatinous egg masses. Nanoparticles are known for their ability to penetrate host barriers [42] and CurNisNp may be no exception to this. Another possibility is that increase in CurNisNp lipophilicity may facilitate its penetration of the snail eggs, thus leading to greater inhibition of hatching in drug exposed groups, when compared with the negative control. Prolonged time of exposure could however undermine reduction in eggs hatching. The death of all the hatched juvenile snails in nanoparticulate exposed groups compared with the negative control group suggests the cumulative effects of the formulation during exposure. The consistent release of the nanoparticulate drug for more than 5-day (120 h) exposure period could be responsible for this cumulative effect.
Although this is the first report on reproductive toxicity of curcumin-nisin nanoparticle in freshwater snails, metallic-nanoparticle induced reproductive toxicity has been observed in molluscs [43], crustaceans [44,45], and marine invertebrates [46]. The snail fecundity rate reduction observed in the formulation exposed groups in our study was similar to observations in some molluscicides and anti-parasitic agents [29,47–49]. The observed reduction in snail egg production might have been due to metabolic changes possibly caused by prolonged exposure of the snail to CurNisNp, which might include destruction of gametogenic cells and damage of hermaphrodite glands possibly resulting from decrease in tissue proteins, DNA damage (apoptosis), or degeneration of cells of these vital organs [29,50,51]. Such a feature is most desirable in a molluscicide of the kind tested here, which is not strongly toxic against the adult snails, as it could be effective in regulating snail populations without necessarily compromising the functional role of the adult snails within the aquatic ecosystem.
Conclusion
It is clear from this study that CurNisNp is a potential molluscicide. It is active against all the snail stages, but with different dynamics of potency. Although the formulation may not prevent hatching of juvenile snails from the egg masses at the pre-hatched stage, it significantly reduced the number of viable juveniles. The adult snails were relatively resistant to the molluscicide, but the significant reduction in their egg laying capacity makes the formulation a potential desirable molluscicide. It is therefore recommended that the formulation be more optimised to give a nanoparticle with a narrow range monodispersed PDI for better drug distribution and eventual molluscicidal activities. More studies on toxicity, stability and photosensitivity of the nanoparticle should also be considered.
Acknowledgments
The nanoparticle used for this study was formulated at the Product Cell Development Laboratory II, National Institute of Immunology.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.World Health Organisation. http://www.who.int/mediacentre/factsheets/fs115/en/index.html. In: Nu115 Fs, editor, 2010.
- 2.van der Werf MJ, de Vlas SJ, Brooker S, Looman CWN, Nagelkerke NJD, et al. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 2003;86:125–139. [DOI] [PubMed] [Google Scholar]
- 3.Howe S, Zöphel D, Subbaraman H, Unger C, Held J, Engleitner T, et al. Lactate as a novel quantitative measure of viability in Schistosoma mansoni drug sensitivity assays. Antimicrob Agents Chemother. 2015;59: 1193–1199. doi: 10.1128/AAC.03809-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrews P, Thyssen J, Lorke D. The biology and toxicology of molluscicides, Bayluscide. Pharmacol Ther. 1983;19: 245–295. [DOI] [PubMed] [Google Scholar]
- 5.Adenusi AA, Odaibo AB. Effects of varying concentrations of the crude aqueous and ethanolic extracts of Dalbergia sissoo plant parts on Biomphalaria pfeifferi egg masses. Afr J Tradit Complement Altern Med. 2009;6(2): 139–149. [PMC free article] [PubMed] [Google Scholar]
- 6.da Silva Filho CRM, de Souza AG, da Conceição MM, da Silva TG, Silva TMS, Ribeiro APL. Avaliação da bioatividade dos extratos de cúrcuma (Curcuma longa L., Zingiberaceae) em Artemia salina e Biomphalaria glabrata. Braz J Pharmacog. 2009;19(4): 919–923. [Google Scholar]
- 7.Adetunji VO, Salawu OT. Efficacy of ethanolic leaf extracts of Carica papaya and Terminalia catappa as molluscicides against the snail intermediate hosts of schistosomiasis. J Med Plant Res. 2010;4(22): 2348–2352. [Google Scholar]
- 8.Salawu OT, Odaibo AB. The molliscicidal effects of Hyptis suaveolens on different stages of Bulinus globosus in the laboratory. Afr J Biotech. 2011;10(50): 10241–10247. [Google Scholar]
- 9.Otarigho B, Morenikeji OA. Molluscicidal effects of aqueous and ethanolic extracts of lemongrass (Cymbopogon citratus) leaf against the different developmental stages of Biomphalaria pfeifferi. New York Sci J. 2012;5(8): 70–77. [Google Scholar]
- 10.Silva L, Souza B, de Almeida Bessa EC, Pinheiro J. Effect of successive applications of the sublethal concentration of Solanum paniculatum in Subulina octona (Subulinidae). J. Natural Prod. 2012;5: 157–167. [Google Scholar]
- 11.Njeh F, Feki H, Koubaa I, Hamed N, Damak M, Ayadi A, et al. Molluscicidal activity of Solanum elaeagnifolium seeds against Galba truncatula intermediate host of Fasciola hepatica: Identification of β-solamarine. Pharm Biol. 2016;54(4): 726–731 doi: 10.3109/13880209.2015.1073332 [DOI] [PubMed] [Google Scholar]
- 12.Nayaka AP, Tiyaboonchai W, Patankar S, Madhusudhan B, Souto EB. Curcuminoids-loaded lipid nanoparticles: a novel approach towards malaria treatment. Colloid Surf B. 2010;81: 263–273. [DOI] [PubMed] [Google Scholar]
- 13.Dauda K, Busari Z, Morenikeji O, Afolayan F, Oyeyemi O, Meena J. et al. Poly-D,L-lactic-co-glycolic acid-based artesunate nanoparticles; formulation, antimalarial and toxicity assessments. J Zhejiang Univ-Sci B (Biomed & Biotechnol), doi: 10.1631/jzus.B12r0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luz PP, Magalhães LG, Pereira AC, Cunha WR, Rodrigues V, Andrade e Silva ML. Curcumin-loaded into PLGA nanoparticles: Preparation and in vitro schistosomicidal activity. Parasitol Res. 2012;110: 593–598. doi: 10.1007/s00436-011-2527-9 [DOI] [PubMed] [Google Scholar]
- 15.Frezza TF, Gremião MPD, Zanotti-Magalhães EM, Magalhães LA, Ribeiro de Souza AL, Allegretti SM. Liposomal-praziquantel: Efficacy against Schistosoma mansoni in a preclinical assay. Acta trop. 2013; 128; 70–75 doi: 10.1016/j.actatropica.2013.06.011 [DOI] [PubMed] [Google Scholar]
- 16.Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of tumeric (Curcuma longa). J Altern Complement Med. 2003;9(1):161–168. doi: 10.1089/107555303321223035 [DOI] [PubMed] [Google Scholar]
- 17.Jones E, Salin V, Williams GW. Nisin and the market for commercial bacteriocins. TAMRC Consumer and Product Research Report No. CP-01-05. 2005. Avaliable online: http://wwww.ageconsearch.umn.edu/bitstream/90779/2/CP%2001%2005%20Nisin%20Report.pdf (accessed on 24 May 2017).
- 18.Ishita C, Kaushik B, Uday B, Ranajit KB. Turmeric and curcumin: Biological actions and medicinal applications. Curr Sci. 2004;87:44–53. [Google Scholar]
- 19.Joo NE, Ritchie K, Kamarajan P, Miao D, Kapila YL. Nisin, an apoptogenic bacteriocin and food preservative, attenuates HNSCC tumorigenesis via CHAC1. Cancer Med 2012;1(3):295–305. doi: 10.1002/cam4.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allam G. Immunomodulatory effects of curcumin treatment on murine schistosomiasis mansoni. Immunobiol. 2009;214: 712–727. [DOI] [PubMed] [Google Scholar]
- 21.Magalhães LG, Machado CB, Morais ER, Moreira EB, Soares CS, da Silva SH, et al. In vitro schistosomicidal activity of curcumin against Schistosoma mansoni adult worms. Parasitol Res. 2009;104: 1197–1201. doi: 10.1007/s00436-008-1311-y [DOI] [PubMed] [Google Scholar]
- 22.Shaikh J, Ankola DD, Beniwal V, Singh D, Ravi-Kumar MNV. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Euro J Pharm Sci. 2009;37: 223–230. [DOI] [PubMed] [Google Scholar]
- 23.Prombutara P, Kulwatthanasal Y, Supaka N, Sramala I, Chareonpornwattana S. Production of nisin-loaded solid lipid nanoparticles for sustained antimicrobial activity. Food Contrl. 2012;24: 184–190. [Google Scholar]
- 24.Madsen H. Effect of calcium concentration on growth and egg laying of Helisoma duryi, Biomphalaria alexandrina, B. camerunensis and Bulinus truncatus (Gastropoda: Planorbidae). J Appl Ecol. 1987;24(3):823–836. [Google Scholar]
- 25.World Health Organisataion. Snail control in the prevention of bilharziasis Mimeograph series, 50, 11, World Health Organization, Geneva, 1965 [PubMed] [Google Scholar]
- 26.World Health Organisation. Report of the scientific working group on plant molluscicides UNDP/World Bank/WHO special programme for Research and Training in Tropical Diseases. World Health Organization, Geneva, 1983 [Google Scholar]
- 27.Olivier L, Haskins WT. The effects of low concentrations of sodium pentachlorophenate on the fecundity and egg viability of Australorbis glabratus. Amer J Trop Med Hyg. 1960;9(2): 199–205. [DOI] [PubMed] [Google Scholar]
- 28.dos Santos AF, Ferraz PAL, Pinto AV, Maria do Carmo FR, Goulart MO, Sant'Ana AEG. Molluscicidal activity of 2-hydroxy-3-alkyl-1, 4-naphthoquinones and derivatives. Inter J Parasitol. 2000;30(11): 1199–1202. [DOI] [PubMed] [Google Scholar]
- 29.Osman GY, Mohamed AZ, Sheir KS, Hassab EL-Nabi SE, Allam AS. Molluscidal activity of Mirazid on Biomphalaria alexandrina snails: biological and molecular studies. Inter J Adv Res. 2014;2(2): 977–989. [Google Scholar]
- 30.Nobbmann ULF. Polydispersity–what does it mean for DLS and chromatography? http://www.materials-talks.com/blog/2014/10/23/polydispersity-what-does-it-mean-for-dls-and-chromatography, 2014. Accessed 24/10/2016
- 31.Srinivasan GP, Delma CR, Elamaran A, Somasundaran ST. Cytoxicity effect of acetone extract of Curcuma longa in brine shrimp, Artemia salina (L). Inter J Curr Biotech. 2014;2(9):1–6. [Google Scholar]
- 32.Adenusi AA, Odaibo AB. Preliminary laboratory assessment of the crude aqueous and ethanolic extracts of Dalbergia sissoo plant parts for molluscicidal, ovicid-al and cercaricidal activities. Trav Med Inf Dis. 2008;6: 219–227. [DOI] [PubMed] [Google Scholar]
- 33.Okeke OC, Ubachukwu PO. Molluscicidal Effects of Talinum triangulare on Bulinus truncatus. Nig J Biotech. 2011;22: 13–16. [Google Scholar]
- 34.Sarquis O, Pieri OS, dos Santos JAA. Effects of Bayluscide on the survival and water-leaving behaviour of Biomphalaria straminea, snail host of schistosomiasis in northeast Brazil. Mem. Inst. Oswaldo Cruz. 1997;92(5): 511–518. [DOI] [PubMed] [Google Scholar]
- 35.Brackenbury TD, Appleton CC. Structural damage to the foot-sole epithelium of Bulinus africanus following exposure to a plant molluscicide. Malacologia 1999;41: 393–401. [Google Scholar]
- 36.Mahobiya P. Study on reproductive performance, survivality mortality of plant glycoside of Abrus precatrious, J Asian Resonance 2013;2(1):50–52. [Google Scholar]
- 37.Webb G. The use of molluscicides in the control of human trematode infections In: The Toxicology of Molluscicides (Webb G., ed.), Pergamon Press, Oxford, 1987;pp. 1–11. [Google Scholar]
- 38.De souza CP, Mendes N, Araújo KN, Katz N. Molluscicidal activity of butanolic extract of Phytolacca dodecandra (endod) on Biomphalaria glabrata. Mem Insti Oswaldo Cruz 1987;82: 345–349. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Guo YH. Study on the effect of bromoacetamide upon the development of snail eggs. J Parasitol Parasitic Dis. 1992;10: 258–262. [PubMed] [Google Scholar]
- 40.Adewunmi CO. Control of snail intermediate hosts of schistosomiasis. Nig J Parasitol. 1993;14: 45–51. [Google Scholar]
- 41.Parashar BD, Kaushik MP, Gupta AK, Swamy RV, Rao KM. Toxicity of some molluscicides to freshwater snail Lymnaea auricularia the vector of animal fasciolasis and to non-target organisms. Proc. Acad. Environ. Biol. 1995;4: 183–187. [Google Scholar]
- 42.Lai SK, O’Hanlon DE, Harrold S, Man ST, Wang YY, Cone R, et al. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc Natl Acad Sci USA 2007;104:1482–1487. doi: 10.1073/pnas.0608611104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ringwood AH, McCarthy M, Bates TC, Carroll DL. The effects of silver nanoparticles on oyster embryos. Marine Environ Res. 2010;69: S49–51. [DOI] [PubMed] [Google Scholar]
- 44.Mendonḉa E, Diniz M, Silva L, Peres I, Castro L, Correia JB, et al. Effects of diamond nanoparticle exposure on the internal structure and reproduction of Daphnia magna. J Hazard Mat. 2011;186:265–71. [DOI] [PubMed] [Google Scholar]
- 45.Seitz F, Bundschuh M, Rosenfeldt RR, Schulz R. Nanoparticle toxicity in Daphnia magna reproduction studies: the importance of test design. Aquatic Toxicol. 2013;126:163–168. [DOI] [PubMed] [Google Scholar]
- 46.Gallo A, Boni R, Buttino I, Tosti E. Spermiotoxicity of nickel nanoparticles in the marine invertebrate Ciona intestinalis (ascidians), Nanotoxicol. 2016;10(8): 1096–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rizk ET. Schistosomaisis control: Evaluation of the molluscicidal activity of a plant extract (Sesbania sesban) against Biomphalaria alexandrina. J Egypt Germ Soc Zool. 1998;27: 91–107. [Google Scholar]
- 48.EL-Ansary AE, Ahmed SA, Aly SA. Antischistosomal and liver protective effects of Curcuma longa extract in Schistosoma mansoni infected mice. Indian J Expt Biol. 2007;45:791–801. [PubMed] [Google Scholar]
- 49.Massoud AM, Habib FS. The effects of myrrh (Commiphora molmol) on the infected snails of Schistosoma sp. and their egg masses: effect on shedding of cercariae and on snail fecundity. J Egypt Soc Parasitol. 2003;33: 585–596. [PubMed] [Google Scholar]
- 50.Metwally A, Janku I, Kemper F, Khayyal M, Ebeid F, Botros S. Effect of schistosomiasis infection on the clearance of phenazone (Antipyrine) in mice. Arzneimittel-Forschung 1990;40: 206–209. [PubMed] [Google Scholar]
- 51.Singh A, Singh DK. Effects of herbal molluscicides and their combinations on the reduction of the snail Lymnaea acuminate. Arch. Environ. Contam. Toxicol. 2004;46: 470–477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.