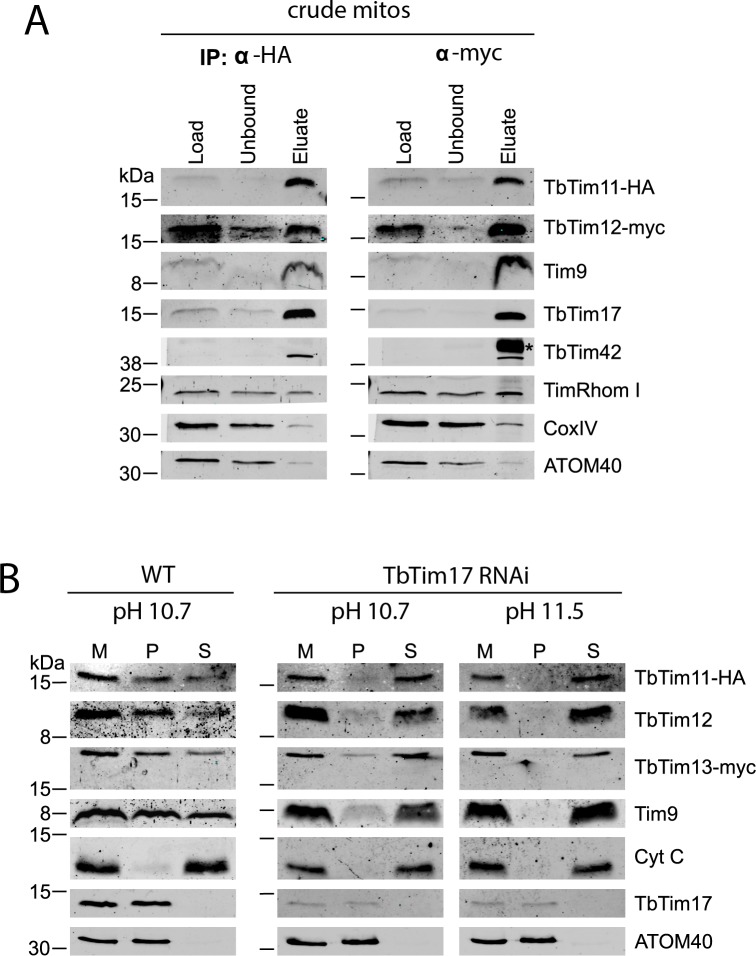
Fig 2. Small Tims are bound to the IM by association with the TIM complex.
A) A cell line co-expressing TbTim11-HA and TbTim12-myc was subjected to co-immunoprecipitation targeting either the HA- (left panel) or the myc-tagged protein (right panel). 5% of the respective lysate (”Load”), 5% of the unbound proteins after IP (“Unbound”) and 100% of the final eluate (“Eluate”) were subjected to SDS-PAGE and western blotting. The blots were probed for the tagged small Tims and the TIM components Tim9, TbTim17, TbTim42 and TimRhom I. ATOM40, the central components of the OM translocase, and the cytochrome oxidase subunit IV (CoxIV) served as controls. The asterisk denotes the co-eluted heavy chain of the anti-myc antibody. B) Alkaline carbonate extraction at low (pH 10.7) and high stringency (pH 11.5) was performed on digitonin-extracted crude mitochondria (M). Mitochondrial transmembrane proteins such as TbTim17 and ATOM40 as well as tightly associated proteins are retained in the resulting pellet fraction (P), while the soluble marker protein cytochrome c (Cyt C) is released to the supernatant (S). Cell lines co-expressing TbTim11-HA and TbTim13-myc in either wildtype (WT) or TbTim17 RNAi background (2 days induced) were used to analyze small Tim fractionation in the presence (left panel) or absence of TbTim17 (right panel).