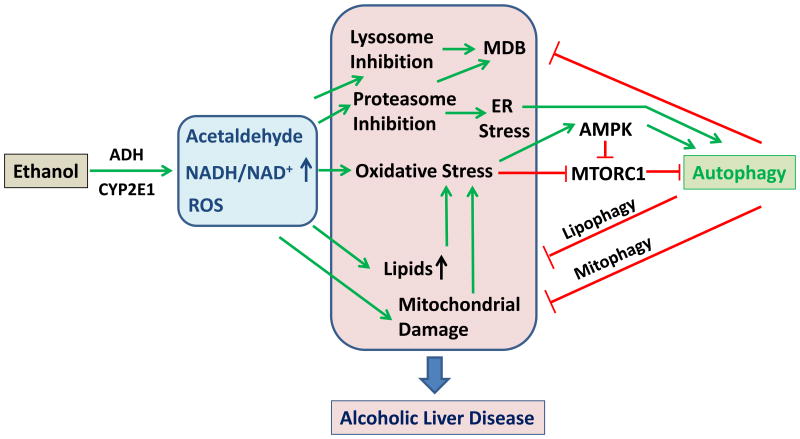
Figure 1. The activation and function of autophagy in alcoholic liver disease.
Ethanol metabolism through alcohol dehydrogenase (ADH), and/or P450 CYP2E1 results in an increased amount of acetaldehyde, an increased NADH/NAD+ ratio and an increased production of reactive oxygen species (ROS). The oxidative stress is further enhanced with the accumulation of lipids and mitochondrial damage, which can cause additional production of ROS and lipid peroxidation. Chronic use of alcohol can also result in proteasome and lysosome inhibition, leading to ER stress and accumulation of SQSTM1/p62-positive ubiquitinated aggregated proteins, known as Malory-Denk body (MDB). These pathological cellular changes contribute to the development of alcohol fatty liver disease. On the other hand, oxidative stress can activate autophagy by increased AMPK activity and decreased MTORC1 activity. Proteasome inhibition can also enhance autophagy via ER stress, although lysosome inhibition can suppress autophagy. Autophagy may protect the liver against ethanol-induced damage by removing MDB, lipids and damaged mitochondria. Pharmacological enhancement of autophagy could thus be an effective and feasible way to treat alcoholic liver disease.