Figure 2.
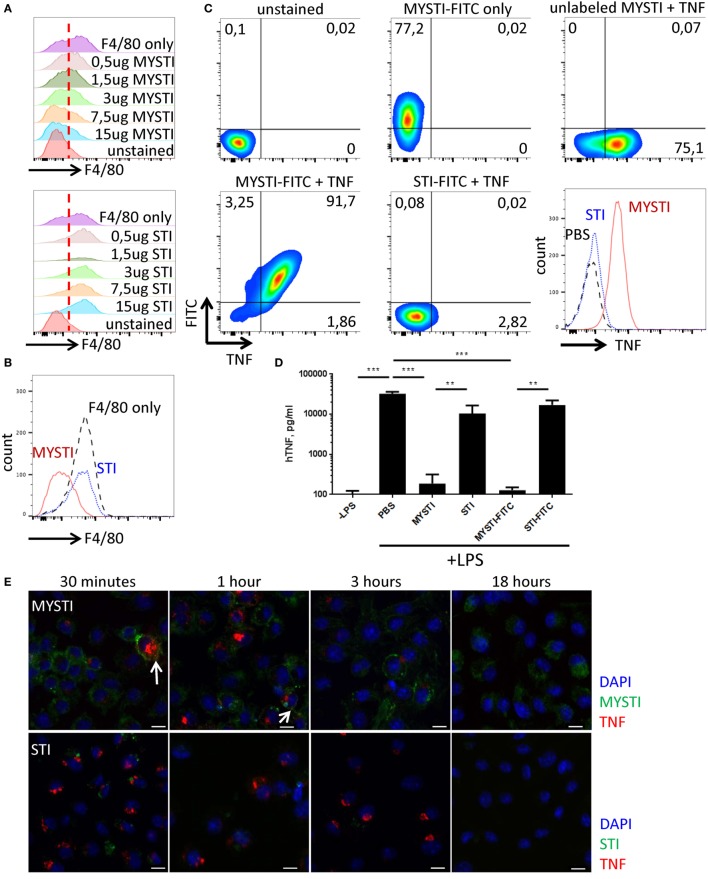
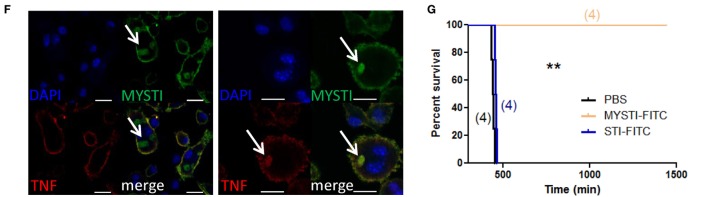
Characterization of MYSTI and STI interaction with macrophages in vitro and in vivo. (A) MYSTI, but not STI, competes with anti-F4/80 antibody for binding sites resulting in reduced staining for F4/80. Staining of macrophages with anti-F4/80 antibody in the presence of indicated concentrations of MYSTI (top panel) or STI (bottom panel). Red dotted line splits F4/80− and F4/80+ cells on the left and on the right, respectively. Briefly, bone marrow-derived macrophages were simultaneously incubated with anti-F4/80 antibody (clone BM8 that competed for binding to F4/80 with anti F4/80 VHH, used in MYSTI) and indicated amounts of MYSTI or STI. All cells were gated as ViabilityDye−CD11b+. (B) Staining of macrophages with anti-F4/80 only or in the presence of MYSTI or STI. Data indicate that MYSTI selectively binds to F4/80. (C) MYSTI, but not STI, binds to the surface of macrophages and retains exogenously added hTNF. Surface staining of macrophages with MYSTI or STI and hTNF. Top row represents unstained or single stained cells as controls. Bottom row represents staining of macrophages with MYSTI-FITC and hTNF (left), STI-FITC and hTNF (middle), and a summarizing histogram of hTNF staining (right). Briefly, bone marrow-derived macrophages were subsequently incubated with MYSTI or STI followed by recombinant human TNF and with anti-hTNF antibody incubations. All cells were gated as VD−CD11b+. (D) MYSTI, but not STI, prevents hTNF release into the culture medium by LPS-stimulated macrophages. BMDM from hTNFKI mice were cultured with MYSTI or STI antibodies or PBS, washed once, and stimulated with 100 ng/ml of LPS from E. coli. Release of hTNF into culture medium was measured 4 h following induction with LPS using Ready-Set-Go ELISA kit (eBioscience). **p < 0.01; ***p < 0.001 in one-way ANOVA. (E) Dynamics of MYSTI and STI staining on LPS-activated macrophages as revealed by confocal microscopy. Briefly, macrophages were activated with 100 ng/ml of LPS for 3 h, followed by incubation with FITC-labeled MYSTI or STI for 15 min, then washed, and fixed at indicated time points. Fixed cells were consequently permeabilized and stained with anti-hTNF Ab labeled with PE. Starting from 30 min of incubation, MYSTI could be detected both on macrophage surface and inside the cells, while weak binding of STI was observed only after 30 min of incubation. Arrows show co-staining of MYSTI and anti-hTNF. Scale bars—10 μm. (F) MYSTI is internalized by macrophages. Confocal microscope images of macrophages stained with MYSTI (green), anti-hTNF (red), and counterstained with DAPI (blue). Briefly, cells were consequently incubated with MYSTI-FITC, recombinant hTNF, and anti-hTNF labeled with PE and then fixed. On each of the two images, top left part represents DAPI staining, top right—MYSTI-FITC, bottom left—anti-hTNF-PE, and bottom right—merged picture. Arrows show internalized MYSTI bound (right image) or not bound to hTNF (left image). Scale bars—20 μm. (G) FITC-labeled MYSTI retains its ability to protect mice in the model of LPS/D-Gal-induced hepatotoxicity. Briefly, mice were injected i.p. with 1.5 mg/kg, STI, or PBS and after 30 min were injected with lethal dose of LPS/D-Gal.