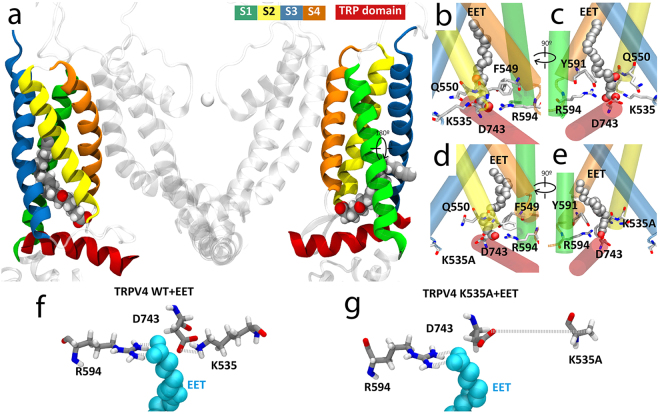
Figure 1.
Predicted model of TRPV4 channel interaction with 5′,6′-EET. (a) TRPV4 structural model showing only two of the four identical subunits (side view). Color-coded transmembrane segments S1-S4, TRP box and EET molecule are highlighted. Images of the predicted EET-binding site in TRPV4-WT (b,c) and TRPV4 K535A (d,e) systems. Residues showing more than 60% of time occupancy are displayed in licorice representation. K535A residue from TRPV4 K535A is displayed in licorice representation even though its interaction is not significant. (f,g) Bottom view detail of the interaction of 5′,6′-EET with residues K535 and R594 in the TRPV4-WT (f) and TRPV4-K535A (g) systems. Note the loss of interaction between mutated residue A535 and both D743 and EET molecule.