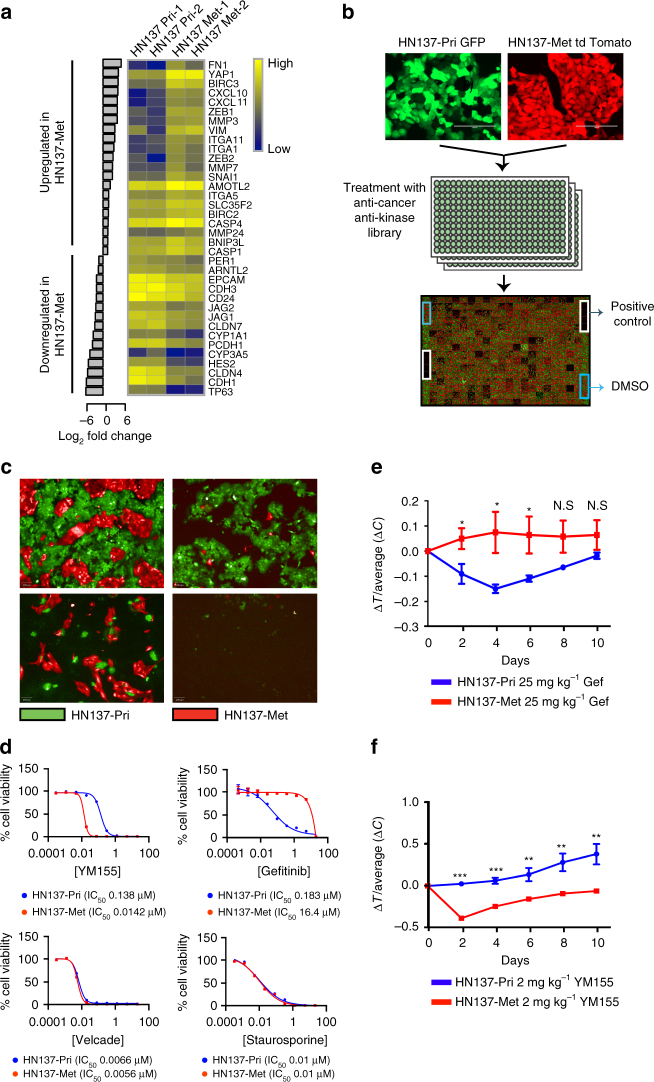
Fig. 2.
Small molecule screen against HN137 PDC revealed patient-specific therapeutic vulnerabilities validates in vivo. a Differentially expressed genes (fold-change >2) in HN137-Met vs. HN137-Pri. The heat map represents (Log10) normalized gene expression data in duplicates. b Schema of the co-culture chemical screen performed in triplicate. c Representative fluorescence images of cells treated with compounds specifically targeting either HN137-Pri (bottom left), HN137-Met (top right) or both (bottom right). Scale bars, 100 μm. d Secondary validation of HN137-Met-specific cytotoxic compound (YM155), HN137-Pri-specific cytotoxic compound (gefitinib) and dual cytotoxic compounds (Velcade and Staurosporine). Experiments were performed at least twice, in triplicates. Cell viability was determined using CellTiter-Glo reagent. Triplicate data, error bars represent mean ± s.d. e Four independent cohorts of male mice (n = 5), bearing tumours in both flanks from HN137-Pri PDX and HN137-Met PDX, were treated with vehicle (control), or 25 mg kg−1 gefitinib (Gef). Note that the HN137-Pri PDX display greater response to gefitinib compared to HN137-Met PDX at earlier days (Days 2-6). Error bars represent mean ± s.e.m. Two-tail Student’s t test was carried out between HN137-Pri and HN137-Met for days 2-10; N.S.: not significant, *P < 0.05. f Four independent cohorts of male mice (n = 5), bearing tumours on both flanks from HN137-Pri PDX and HN137-Met PDX, were treated with 2 mg kg−1 of YM155, compared to vehicle (control). A significant anti-tumour effect was observed for YM155 treatment in HN137-Met PDX while HN137-Pri PDX did not display significant sensitivity to YM155. Two-tail Student’s t test was carried out between HN137-Pri and HN137-Met for days 2-10; *P < 0.05, **P < 0. 01, ***P < 0. 001