Supplemental digital content is available in the text.
Abstract
Background
Whether injury-related molecules in urines of individuals with ischemia-reperfusion injury (IRI) are independent predictors of graft outcomes and provide additional information compared with usual risk factors remains to be established.
Methods
We explored a cohort of 244 kidney transplant recipients who systematically had a urine collection 10 days after transplantation. The injury-related markers kidney injury molecule-1 (KIM-1) and angiogenin (ANG) levels in urines were measured. We determined the prognostic values of these markers on graft outcomes.
Results
Urinary KIM-1 and ANG concentrations were strongly correlated to each other and were significantly and independently associated with cold ischemia time, delayed graft function, and plasma creatinine 10 days after transplantation, indicating that these markers reflect the severity of IRI. However, urinary ANG and KIM-1 were not predictive of histological changes on protocol biopsies performed 3 and 12 months after transplantation. Finally, urinary ANG and urinary KIM-1 were not associated with graft survival.
Conclusions
Together, our results indicate that, in a cohort of 244 kidney transplant recipients, urinary ANG and KIM-1 levels in a single measurement 10 days after transplantation reflect the severity of IRI after kidney transplantation, but are neither independent predictors of renal function, histological changes and graft survival.
It is acknowledged that the very early period that follows kidney transplantation is critical for the future of the allograft because ischemia-reperfusion injury (IRI) shapes the immune system toward an adaptive response against alloantigens, hence promoting alloimmunity and rejection.1-4 The acute phase of the injury is also susceptible to activate healing processes embedded in inflammatory and fibrogenic reactions that likely lead to irreversible sequelae, such as tubular atrophy and interstitial fibrosis.5-7 In line with this, the intensity of the injury could negatively impact kidney allograft outcomes, including survival.8,9
A better delineation of the features that best anticipate the forthcoming events may require the identification of the multiple biological processes that are activated in response to IRI at the molecular level, for example, adaptive responses to hypoxia (eg, the hypoxia inducible factor-1α pathway), oxidative stress (eg, the nuclear factor [erythroid-derived 2]-like pathway), or the endoplasmic reticulum (ER) stress response.10 Supporting this, a number of signatures related to the molecular mechanisms of acute kidney injury (AKI) have been characterized, and the clinical relevance of their predictive values has been emphasized,5,11,12 and may even be targeted to improve outcomes.13,14 The detection and quantification of markers reflecting the activation of specific biological programs in response to kidney insults may provide information on their intrinsic relevance at the clinical level.15 However, 1 key issue that remains unresolved in this context is the extent to which the fidelity of the markers for the biological process they are supposed to reflect is important for their predictive value, and if noninvasive markers, especially those found in urines, have to closely reflect the specific biological pathway which is at the origin of their production to be valuable predictors of future events. A part of the answer comes from the fact that serum creatinine and proteinuria, which do not in any way reflect a peculiar process, but rather the dysfunction of the organ as a whole, are in general effective biological predictors of graft outcomes.8,16,17 In addition, a challenging issue for emerging biomarkers associated with AKI lies in their ability to provide additional information to well-established clinical risk factors and risk markers.
We recently identified the secreted ribonuclease angiogenin (ANG) as an integral component of the ER stress response in the kidney upon AKI, and that is expressed under the control of the inositol-requiring enzyme 1α/spliced X-Box Binding Protein (sXBP1) pathway, thereby reflecting the activation of the unfolded protein response in the kidney.18,19 A body of work lends support to the conclusion that ER stress may play a role in modulating immunogenic cell death and fibrogenic process that are probably relevant in transplant settings2,20 To evaluate if monitoring of the activation of the inositol-requiring enzyme1α-spliced X-Box Binding Protein pathway upon IRI yields clinically relevant information on kidney allograft outcomes, we evaluated the ability of urinary ANG to predict graft histology, function and survival in a cohort of 244 kidney transplant recipients (KTR) upon recovery from IRI (10 days after transplantation). We compared the performances of urinary ANG with those of kidney injury molecule 1 (KIM-1), a scavenger receptor for apoptotic tubular cells, which reflects programmed cell death of tubular cells, and may be clinically relevant for predicting kidney transplantation outcomes. In IRI and nephrotoxic AKI, KIM-1 expression correlated with the severity of the injury and seems to have prognostic value in the early posttransplantation period.21,22
Our results indicate that both urinary ANG and KIM-1 capture the severity of AKI in a similar extent. However, neither urinary KIM-1 nor ANG levels predict graft histology and survival over the follow-up period, whereas donor age and plasma creatinine are the best independent predictors of graft further outcomes.
MATERIALS AND METHODS
Population
Patient and urine samples used in this study have been part of a previously published analysis.23 Four hundred five consecutive kidney transplantations performed at our center from January 2010 to June 2012 were screened; patients with human immunodeficiency virus and/or hepatitis C virus infection were excluded. Other exclusion criteria consisted of primary nonfunction or early graft loss, death within the first 6 months, early loss of follow-up, or inclusion in another protocol. These patients were excluded due to the design of the study that initially hypothesized that the longitudinal assessment of urine chemokine levels predicts subsequent rejection in apparently stable kidney recipients.23 One hundred five KTRs were then excluded, and 300 were included. The flow chart is depicted in Zhang et al.23 Posttransplantation, urine was collected on day 10 for 244 of the 250 available 10 days after transplantation in individuals initially included in this longitudinal, single-center cohort study. The study was approved by the ethics committee of Ile-de-France XI (13016), and all of the participating patients provided written informed consent.
Urine Protein Analyses
Urine samples were centrifuged at 1000g for 10 minutes within 4 hours of collection. The supernatant was collected after centrifugation and stored with protease inhibitors at −80°C. The urine samples used in this study have been part of a previously published analysis.23 Urinary concentrations of ANG (uANG) and urinary concentrations of KIM-1 (uKIM1) were quantified using the quantikine human ANG and KIM-1 immunoassays (RD Systems), according to the manufacturer's protocol. The performances of the ELISA protocol for the dosage of ANG in urine follows the French guidelines for methods validations (derived from the ISO 15189:2012, which specifies requirements for quality and competence in medical laboratories, and has been published in Tavernier et al.11 Because the uANG and uKIM1 were strongly correlated with their respective ratio with the corresponding urinary creatinine (Figure S1, SDC, http://links.lww.com/TXD/A51), we did not adjust the value for urinary creatinine. Because the distribution of the values of urinary ANG and KIM-1 was skewed, we log-transformed the values to obtain a Gaussian distribution. Therefore, uANG and uKIM1 refer to log-transformed urinary concentration of ANG and KIM-1.
Biopsies
Protocol biopsies were performed at months 3 and 12 posttransplantation. Kidney allograft biopsies were classified using the Banff 2007 update of the Banff 1997 classification (15) by MR.
Statistical Analysis
Continuous variables are presented as means and standard deviation, and categorical variables as presented as proportions. For variance analysis of continuous variables in different groups, Student t test and Kruskal-Wallis with Dunn multiple comparison test were used. Dichotomous variables were compared using the χ2 test. Multiple linear regression model using standard least squares and ordinal logistic regression models were built according to the variable to explain. We analyzed the kidney biopsies and allograft function in excluding from the cohort the 58 KTR from living donors because measuring markers of IRI in this subgroup appeared less relevant. Among the 244 KTR, we evaluated the rate of graft failure according to expanded criteria donor (ECD) status, living donor status, delayed graft function (DGF) status, and in the population of living donors only according to uANG and uKIM1 medians, using Kaplan-Meier curves and compared them with a Log-Rank test. In case of death with a functioning graft, we censored graft survival at the time of death. Statistical analyses were performed using JMP.10 (SAS software). All tests were 2-sided, and P values less than 0.05 were considered to indicate significance.
RESULTS
uANG and uKIM1 Reflect the Severity of Ischemic Injury
We first thought to determine if and how urinary ANG and KIM-1 are reflective of AKI in kidney allografts after IRI. To do this, we measured uANG and uKIM1 (see Methods for definition) of 244 KTRs 10 days after transplantation, whose clinical characteristics are listed in Table 1. The notable specificities of this cohort are that 23% received a kidney from a living donor, 50% from an ECD, and nearly 20% of the recipients had preformed donor-specific antibodies (DSA). All received an induction therapy with either antithymoglobulins in 56% of the cases or anti-CD25 antibodies in 44% of the cases. The clear majority (80%) received tacrolimus, mycophenolate mofetil, and corticosteorids.
TABLE 1.
Demographic and clinical characteristics of the cohort of KTRs who had a urinary sample analysis 10 days after transplantation
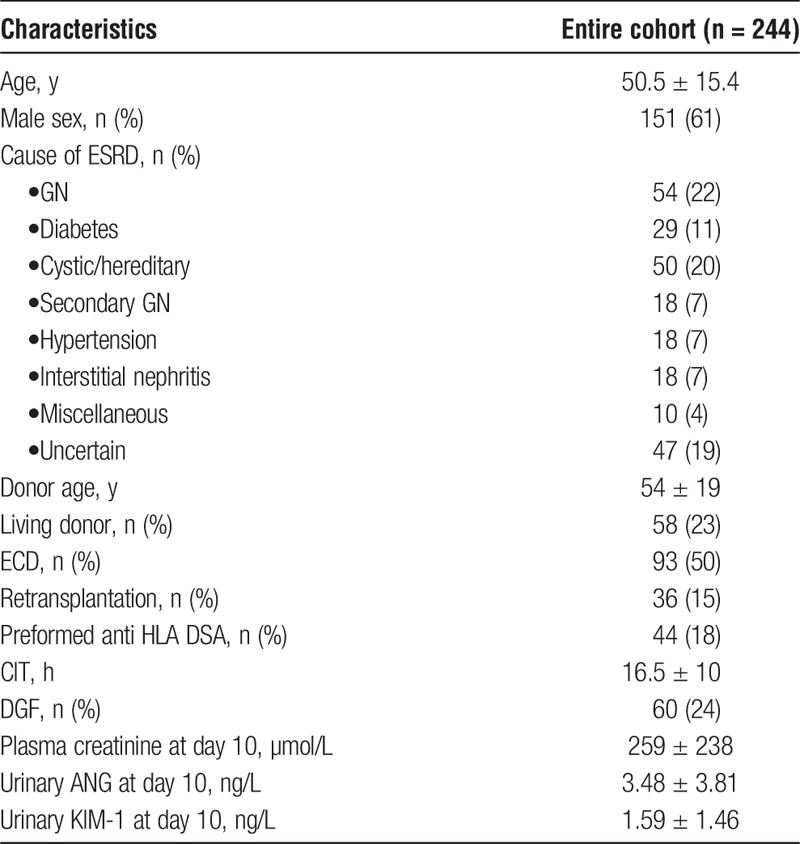
Ten days after transplantation, uANG and uKIM1 were highly correlated to each other (R2 = 0.27, P < 0.001) (Figure 1A) indicating that similar processes support their production. In line with this, uANG and uKIM1 were associated with parameters directly reflecting the severity of ischemic AKI. For example, uANG and uKIM1 were higher in KTRs with higher plasma creatinine values (Figure 1B), with DGF and deceased donor (Figures 1C and D) and in KTR having had numerous dialysis sessions (Figure 1E). In addition, the use of IvIG as an induction therapy was associated with higher values of uANG and uKIM1, possibly reflecting mild tubular injury (Figure 1F). Consistent with the fact that uANG and uKIM1 do not refer to the same pathogenic process upon AKI, cold ischemia time (CIT) did not influence uANG whereas it was strongly associated with uKIM1 (Figure 1G). Because all these parameters associated with uANG and uKIM1 are highly covariant, we generated models predicting uANG and uKIM and integrating CIT, DGF, IvIG use and plasma creatinine at day 10 (Figure 1H), and we observed that uANG was independently associated with plasma creatinine at day 10 and IvIG use, thereby directly reflecting AKI, whereas uKIM1 was mostly influenced by CIT. Together, these results indicate that uANG and uKIM1 are markers of AKI induced by ischemia-reperfusion. In addition, uANG appears to be less influenced by CIT compared to uKIM1.
FIGURE 1.
Biochemical characterization of urinary ANG. A, Scatter plot representing the bivariate fit of uKIM1 values by uANG values in this whole cohort (n = 244). B, Box and whiskers plots representing the distribution of plasma creatinine according to uANG and uKIM1 median, Student t test. C, Box and whiskers plots representing the distribution of uANG and uKIM median according to DGF, Student t test. D, Box and whiskers plots representing the distribution of uANG and uKIM according to the live status of the donor, Student t test. E, Box and whiskers plots representing the distribution of uANG and uKIM according to the number of dialysis sessions, Kruskal-Wallis test. F, Box and whiskers plots representing the distribution of uANG and uKIM according to IvIG use, Student t test. G, Box and whiskers plots representing the distribution of CIT according to uANG and uKIM1 median, Student t test. H, Multiple linear regression analysis (standard least squares model) between uKIM (up) and uANG (down) and CIT, IvIG induction (no), plasma creatinine and DGF (no). Estimate values give the estimate of the model coefficients for each term. The t ratio is the ratio of the estimate to its standard error and tests whether the true value of the parameter is zero.
uKIM and uANG Are Not Predictive of Kidney Allograft Pathology
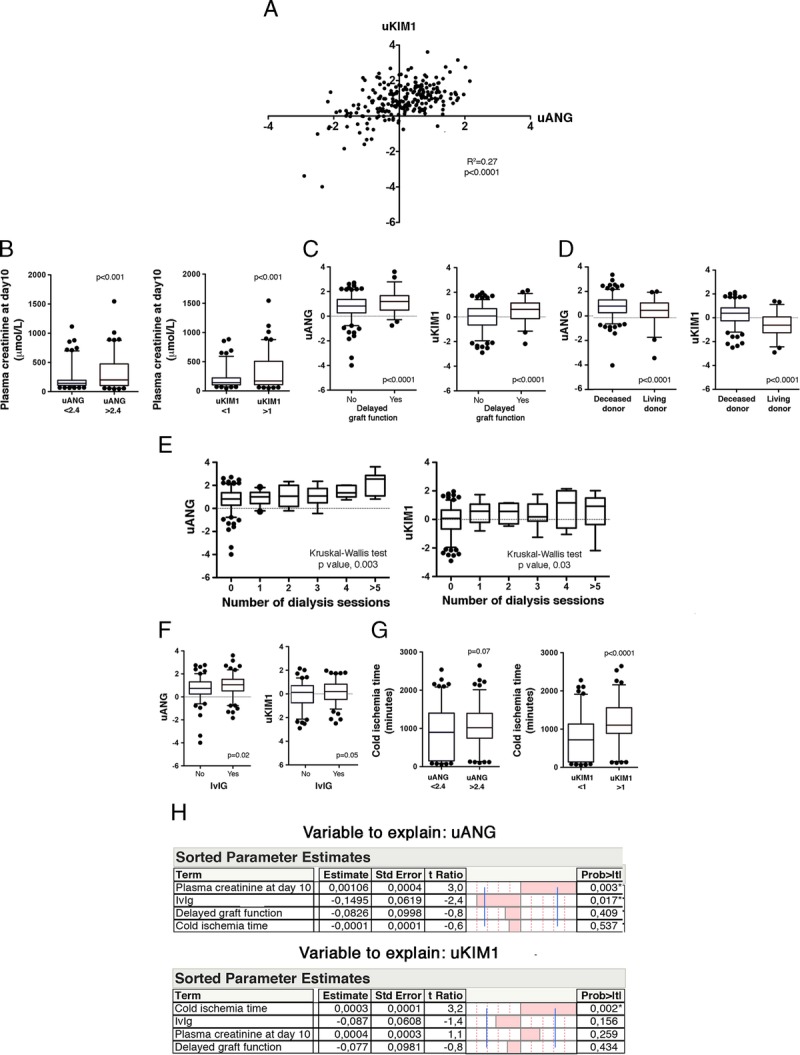
Because uANG and uKIM1 are markers of the magnitude of the injury imposed by ischemia-reperfusion, and which may trigger alloimmunity and fuel chronic structural deterioration, we tested whether these markers could be predictive of future histological changes. We analyzed the protocol kidney biopsies performed 3 months after transplantation in excluding from the cohort the 58 KTR from living donors because measuring markers of IRI in this sub group appeared less relevant. Therefore, 152 protocol biopsies were analyzed to evaluate the distribution of and uKIM1 according to acute and chronic scores (i + t, g + ptc and ci + ct) as listed in the Banff classification24 (Figure 2). When measured 10 days after transplantation, uANG and uKIM1 were not associated with histological markers of T cell–mediated rejection (i + t), acute humoral rejection (g + ptc) or interstital fibrosis-tubular atrophy (ci + ct) occurring during the 3 months after transplantation. We also measured the association of uKIM1 and uANG with histological findings in protocol biopsies performed 1 year after transplantation (n = 142 biopsies), and we found that uANG and uKIM1 were not associated with (i + t), (g + ptc) and (ci + ct) scores (not shown). Together, these results indicate that neither uANG nor uKIM1, which reflect the severity of AKI, are predictive markers of the occurrence of histological changes after transplantation.
FIGURE 2.
uKIM and uANG are not predictive of kidney allograft pathology. A, B, C, Box and whiskers plots representing the distribution of uANG (left) and uKIM (right) per the Banff (i + t) scores (A), (ci + ct) score (B), (g + cpt) score (C) in the cohort of patients, living donors excluded (n = 152), Kruskal-Wallis test.
uANG and uKIM1 Are Not Predictive of Allograft Function
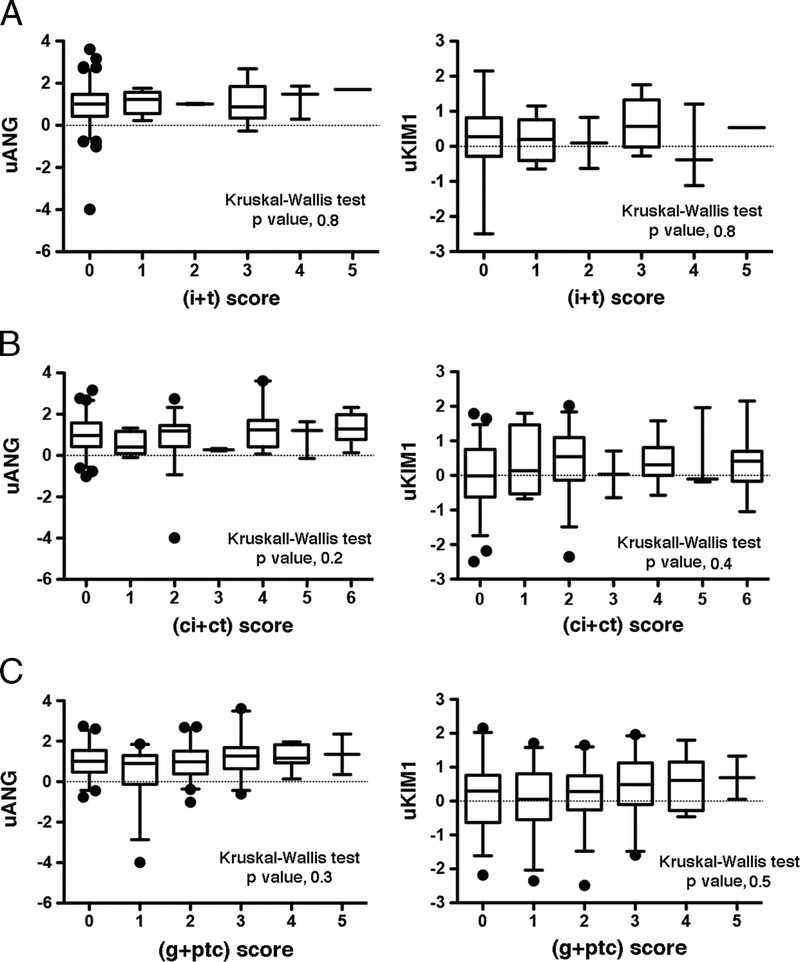
Because renal function only partially reflects histological findings and structural deterioration that may occur within the allograft, we evaluated whether uANG and uKIM1 could allow for predicting allograft function. To do this, we generated multivariate models predictive of renal function (glomerular filtration rate estimated with the MDRD formula, estimated glomerular filtration rate [eGFR]), 3 and 12 months after transplantation, and we tested clinical parameters that individually impact graft function in the KTRs (in excluding living donors) with a P value less than 0.2. Among these parameters, uANG (P = 0.12), CIT (P = 0.2), DGF (P = 0.004), ECD (P < 0.0001), donor age (P < 0.0001), and biopsy proven acute rejection (P = 0.19), but not uKIM (P = 0.7), were integrated in the model predictive of eGFR at month 3; and for the model predicting renal function at month 12, we integrated uANG (P = 0.05), DGF (P = 0.02), ECD (P < 0.0001), donor age (P < 0.0001), uKIM1 (P = 0.11), and biopsy proven acute rejection (P = 0.07) (Figures 3A and B). The only parameter that independently predicted eGFR was donor age, and neither uANG or uKIM1 were independently associated with renal function, as did parameters that reflect AKI, such as CIT or DGF.
FIGURE 3.
uANG and uKIM1 are not predictive of allograft function. A, Multiple linear regression analysis (standard least squares model) between eGFR 3 months after transplantation and parameters associated with eGFR at month 3 with P < 0.2 in univariate analysis: donor age, DGF (no) extended criteria donor (no), biopsy proven acute rejection (no), uANG and CIT. Living donors excluded (n = 186) Estimate values give the estimate of the model coefficients for each term. The t ratio is the ratio of the estimate to its standard error, and tests whether the true value of the parameter is zero. B, Multiple linear regression analysis (standard least squares model) between eGFR 12 months after transplantation and parameters associated with eGFR at month 12 with P < 0.2 in univariate analysis: donor age, biopsy proven acute rejection (no), DGF (no), uANG, extended criteria donor (no), and uKIM1. Living donors excluded (n = 147). C, Multiple linear regression analysis (standard least squares model) between eGFR 3 months after transplantation and biological parameters associated with eGFR at month 3 with P < 0.2 in univariate analysis: plasma creatinine, de novo DSAs, preformed donor serum antibodies and uANG. Living donors excluded (n = 186). D, Multiple linear regression analysis (standard least squares model) between eGFR 12 months after transplantation and biological parameters associated with eGFR at month 12 with P < 0.2 in univariate analysis: plasma creatinine, de novo DSAs, preformed donor serum antibodies and uANG. Living donors excluded (n = 152).
We also evaluated the performances of uANG and uKIM1 in predicting renal function in models built with biological markers that would predict renal function and which were associated with eGFR with a P value less than 0.2 in univariate analyses: at month 3 in including plasma creatinine at day 10 (P < 0.0001), preformed DSA (P = 0.2), de novo DSA (P = 0.03), and uANG (P = 0.12), but not uKIM1 (P = 0.7) (Figure 3C), and at month 12 in including plasma creatinine at day 10 (P < 0.0001), preformed DSA (P = 0.1), de novo DSA (P = 0.0007), uANG (P = 0.05), and uKIM1 (P = 0.1) (Figure 3D). In these models, the best independent predictor of renal function at both month 3 and month 12 was plasma creatinine 10 days after transplantation. In addition, de novo DSAs were independently associated with lower eGFR 12 months after transplantation. Overall, these results indicate that uANG and uKIM1 are unable to predict kidney allograft function.
uANG and uKIM1 Are Not Predictive of Graft Outcomes
Finally, we evaluated the prognostic values of uANG and uKIM on allograft survival at the last follow-up visit. The mean follow-up period was 43 ± 13 months, and the overall graft failure rate was 7%. As expected, the graft survival of KTRs who received an ECD kidney was significantly lower compared with those who received a kidney from a standard criteria donor, and conversely, graft survival was higher in KTRs having received a kidney from a living donor (Figures 4A and B). Markers of initial AKI, including DGF (Figure 4C), did not impact graft survival, as did high levels of uANG or uKIM1. Overall, these results support a model in which IRI fosters ANG and KIM-1 production in urines during the early period after transplantation, and likely reflects the severity acute renal lesions, but is not predictive of further graft failure.
FIGURE 4.
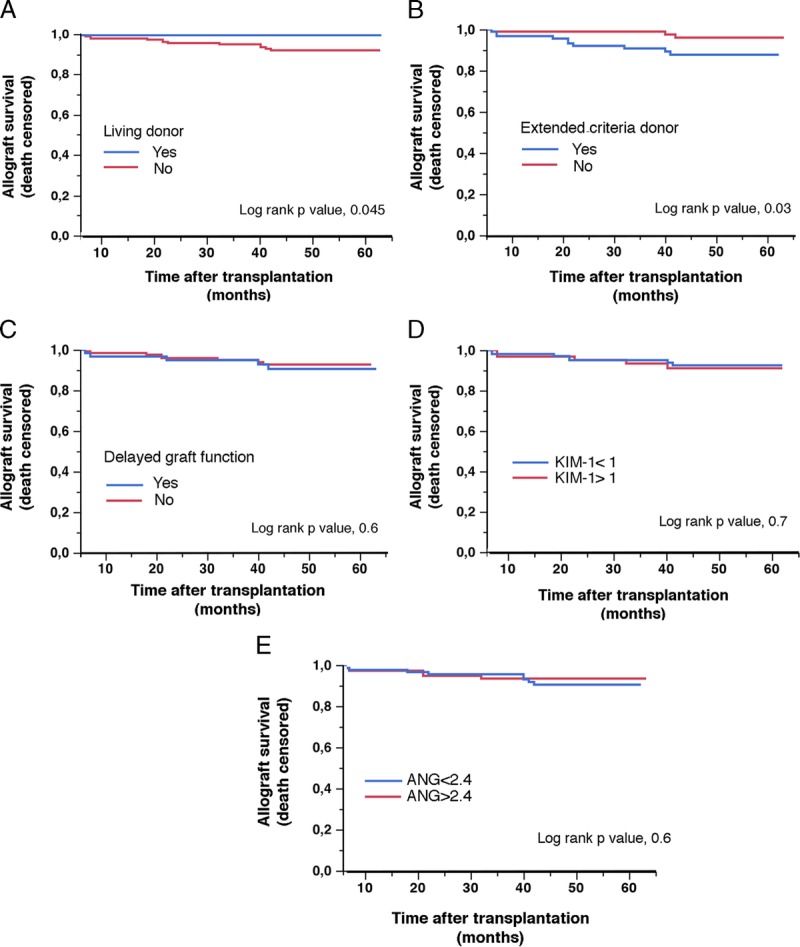
uANG and uKIM1 are not predictive of graft outcomes. Kaplan-Meier curves for the association between graft survival in the cohort of 244 KTRs according to living donor (A), extended criteria donor (B), DGF (C), uANG median (D) and uKIM median (E). Living donors were excluded from uANG and uKIM1 analyses.
DISCUSSION
We undertook this study to test if the quantification of molecules produced by the renal epithelium in urines after IRI and that reflect a particular biological process could provide clinically relevant information on the occurrence of future events. Our results indicate that ANG, which reflect the activation of the ER stress response, and KIM-1, which is more related to apoptotic cell death, are markers of AKI, and reflect the severity of the injury, but do not carry any informative value on the occurrence of histological changes, renal function and graft survival.
The rational of our study was based on the fact IRI is predicted to create an immune environment that facilitates antigen presenting cells activation ultimately leading to alloimmunity on 1 side and may promote definitive structural lesions on the other side, which would ultimately impact renal function and graft survival. A large body of clinical and experimental evidence supports these assumptions.8,25 Actually, the impact of IRI on graft future is still debated, and large epidemiological studies have showed that CIT-related DGF have no or limited impact on the future of graft outcomes.26-28 In fact, the extent that IRI-associated DGF affects long-term graft function and survival is not clear, and differing views exist about the impact of AKI upon long-term outcomes. There are arguments in the literature supporting the concept that AKI causes transient renal dysfunction followed by healing and recovery, and that long-term outcomes are determined by donor and recipients factors, as it is the case in our study.15 A reason for our negative results, and which appears throughout our study, is that IRI have rather self-limited consequences on the kidney transplant, both in terms of immunogenicity or fibrogenesis. Indeed, in our cohort, none of the parameters associated with IRI-induced AKI, the most clinically relevant being DGF, were independent predictors of renal function, interstital fibrosis-tubular atrophy, rejection or renal survival, in multivariate analyses. Supporting this, molecular phenotyping of the changes that occur upon IRI in kidney transplant revealed self-limited repair processes which intensity is reflective of the severity of the injury, but are not predictive of further events.29-31 On the other hand, the clinical parameters that most strongly influenced renal function, histology and survival were related the medical situations reflecting preexisting chronic lesions, namely donor age and ECD, which likely outweighed the impact of parameters associated with AKI. Because serum creatinine is the primary determinant of eGFR, it makes sense that the best predictor of eGFR predictor is serum creatinine. This is 1 of the basic problems with using allograft function as an outcome for biomarker studies.
Our results also raise the issue of the design of biomarkers and their role in the management of KTR.32 Only very few land to the clinic and many have been tested in the early period, and it may be somewhat frustrating to admit that serum creatinine and proteinuria remains the most powerful prognostic markers of long-term prognosis, which indicates that markers of “global” tissue injury are more predictive of the outcomes compared with markers reflecting a precise biological process activated in response to injury. This consideration is consistent with the fact that the impact of clinical factors reflecting the quality of the kidney allograft, such as donor age, or AKI, such as eGFR, is so strong that the window for an additive informative value of many of the markers tested is too small to be relevant. In addition, markers of injury are, by definition, not modifiable, compared with clinical factors, which make them less valuable in a therapeutic point of view.
In conclusion, we have demonstrated that the prognostic value of urinary KIM-1 and ANG as markers of AKI 10 days after transplantation and reflecting specific pathophysiological process related to tissue injury is not significant. However, it would take a larger samples size to definitively prove that these biomarkers are not clinically relevant.
Supplementary Material
Footnotes
Published online 16 August, 2017.
D.A. and N.P. are cosenior authors.
The authors declare no conflicts of interest.
This work was funded by grants from the Institut National de la Santé et de la Recherche Médicale (INSERM), la Fédération Nationale pour l’Aide aux Insuffisants Rénaux (FNAIR) et l’Agence Nationale de la Recherche (ANR). D.A. is supported by the Centaure Network, and the Fonds Emmanuel Boussard.
Q.T. performed ELISA dosages. C.T. and L.M. managed the biocollection. M.R. performed analyses of the kidney biopsies. D.A. managed the cohort, designed the study, and provided critical advices. N.P. designed and supervised the study and wrote the article.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Giblin L, O'Kelly P, Little D, et al. A comparison of long-term graft survival rates between the first and second donor kidney transplanted—the effect of a longer cold ischaemic time for the second kidney. Am J Transplant. 2005;5:1071–1075. [DOI] [PubMed] [Google Scholar]
- 2.Land WG, Agostinis P, Gasser S, et al. DAMP-induced allograft and tumor rejection: the circle is closing. Am J Transplant. 2016;16:3322–3337. [DOI] [PubMed] [Google Scholar]
- 3.Land WG, Agostinis P, Gasser S, et al. Transplantation and damage-associated molecular patterns (DAMPs). Am J Transplant. 2016;16:3338–3361. [DOI] [PubMed] [Google Scholar]
- 4.Opelz G, Döhler B. Multicenter analysis of kidney preservation. Transplantation. 2007;83:247–253. [DOI] [PubMed] [Google Scholar]
- 5.Karim AS, Reese SR, Wilson NA, et al. Nox2 is a mediator of ischemia reperfusion injury. Am J Transplant. 2015;15:2888–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim J, Seok YM, Jung KJ, et al. Reactive oxygen species/oxidative stress contributes to progression of kidney fibrosis following transient ischemic injury in mice. Am J Physiol Renal Physiol. 2009;297:F461–F470. [DOI] [PubMed] [Google Scholar]
- 7.Kuypers DR, Chapman JR, O'Connell PJ, et al. Predictors of renal transplant histology at three months. Transplantation. 1999;67:1222–1230. [DOI] [PubMed] [Google Scholar]
- 8.Debout A, Foucher Y, Trebern-Launay K, et al. Each additional hour of cold ischemia time significantly increases the risk of graft failure and mortality following renal transplantation. Kidney Int. 2015;87:343–349. [DOI] [PubMed] [Google Scholar]
- 9.Ponticelli CE. The impact of cold ischemia time on renal transplant outcome. Kidney Int. 2015;87:272–275. [DOI] [PubMed] [Google Scholar]
- 10.Zuk A, Bonventre JV. Acute kidney injury. Annu Rev Med. 2016;67:293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tavernier Q, Mami I, Rabant M, et al. Urinary angiogenin reflects the magnitude of kidney injury at the infrahistologic level. J Am Soc Nephrol. 2017;28:678–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan Y, Xiao W, Lee K, et al. Inhibition of reticulon-1A-mediated endoplasmic reticulum stress in early AKI attenuates renal fibrosis development. J Am Soc Nephrol. 2017;28:2007–2021. doi: 10.1681/ASN.2016091001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El Karoui K, Viau A, Dellis O, et al. Endoplasmic reticulum stress drives proteinuria-induced kidney lesions via lipocalin 2. Nat Commun. 2016;7:10330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapitsinou PP, Sano H, Michael M, et al. Endothelial HIF-2 mediates protection and recovery from ischemic kidney injury. J Clin Invest. 2014;124:2396–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halloran PF, Famulski KS, Reeve J. Molecular assessment of disease states in kidney transplant biopsy samples. Nat Rev Nephrol. 2016;12:534–548. [DOI] [PubMed] [Google Scholar]
- 16.Naesens M, Lerut E, Emonds MP, et al. Proteinuria as a noninvasive marker for renal allograft histology and failure: an observational cohort study. J Am Soc Nephrol. 2016;27:281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schanstra JP, Zürbig P, Alkhalaf A, et al. Diagnosis and prediction of CKD progression by assessment of urinary peptides. J Am Soc Nephrol. 2015;26:1999–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mami I, Tavernier Q, Bouvier N, et al. A novel extrinsic pathway for the unfolded protein response in the kidney. J Am Soc Nephrol. 2016;27:2670–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mami I, Bouvier N, El Karoui K, et al. Angiogenin mediates cell-autonomous translational control under endoplasmic reticulum stress and attenuates kidney injury. J Am Soc Nephrol. 2016;27:863–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inagi R, Ishimoto Y, Nangaku M. Proteostasis in endoplasmic reticulum—new mechanisms in kidney disease. Nat Rev Nephrol. 2014;10:369–378. [DOI] [PubMed] [Google Scholar]
- 21.Zhang PL, Rothblum LI, Han WK, et al. Kidney injury molecule-1 expression in transplant biopsies is a sensitive measure of cell injury. Kidney Int. 2008;73:608–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nijboer WN, Schuurs TA, Damman J, et al. Kidney injury molecule-1 is an early noninvasive indicator for donor brain death-induced injury prior to kidney transplantation. Am J Transplant. 2009;9:1752–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rabant M, Amrouche L, Morin L, et al. Early low urinary CXCL9 and CXCL10 might predict immunological quiescence in clinically and histologically stable kidney recipients. Am J Transplant. 2016;16:1868–1881. [DOI] [PubMed] [Google Scholar]
- 24.Haas M, Sis B, Racusen LC, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2013;14:272–283. [DOI] [PubMed] [Google Scholar]
- 25.Perico N, Cattaneo D, Sayegh MH, et al. Delayed graft function in kidney transplantation. Lancet. 2004;364:1814–1827. [DOI] [PubMed] [Google Scholar]
- 26.Kayler LK, Srinivas TR, Schold JD. Influence of CIT-induced DGF on kidney transplant outcomes. Am J Transplant. 2011;11:2657–2664. [DOI] [PubMed] [Google Scholar]
- 27.Kayler LK, Magliocca J, Zendejas I, et al. Impact of cold ischemia time on graft survival among ECD transplant recipients: a paired kidney analysis. Am J Transplant. 2011;11:2647–2656. [DOI] [PubMed] [Google Scholar]
- 28.Terasaki PI. Cold ischemia time–time to rethink the risk for kidneys? Am J Transplant. 2011;11:2551–2552. [DOI] [PubMed] [Google Scholar]
- 29.Einecke G, Reeve J, Sis B, et al. A molecular classifier for predicting future graft loss in late kidney transplant biopsies. J Clin Invest. 2010;120:1862–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Venner JM, Famulski KS, Reeve J, et al. Relationships among injury, fibrosis, and time in human kidney transplants. JCI Insight. 2016;1:e85323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Famulski KS, de Freitas DG, Kreepala C, et al. Molecular phenotypes of acute kidney injury in kidney transplants. J Am Soc Nephrol. 2012;23:948–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menon MC, Murphy B, Heeger PS. Moving biomarkers toward clinical implementation in kidney transplantation. J Am Soc Nephrol. 2017;28:735–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


