Abstract
To quantify the mechanical contribution of posterior ligamentous structures to the stability of thoracolumbar compression fractures.
Twelve fresh human T11–L3 spinal specimens were harvested in this study. The 1/3 L1 vertebral body was resected in a wedged shape. After the preinjury had been created, the specimens were subjected to flexion–compression to create a fracture model. Resection of the ligaments was performed in a sequential manner from the bilateral facet capsule ligament (FCL), interspinous ligament, and supraspinous ligament (SSL) to the ligamentum flavum at the T12–L1 level. Then, for the intact specimen, fracture model, and ligament disruption steps, the range of motion (ROM) and neutral zone (NZ) of T12–L1 and L1–L2 were collected for each simulated movement.
Sequential transection of the posterior ligamentous complex (PLC), ROM, and NZ were increased in all movements at the T12–L1 segment. In the flexion–extension (FE), the ROM and NZ demonstrated significant increases after the fracture model and resection of SSL and LF. In lateral bending (LB), the ROM increased after the fracture and removal of the LF, while the NZ showed a slight increase. In axial rotation, the fracture model and removal of the LF resulted in a significant increase in the ROM, and the NZ showed a slight change after step reduction. For the L1–L2 segment, resection of the FCL led to an increased ROM in LB.
With rupture of SSL or LF, the stability of the segment decreased significantly compared with the intact and fracture model, particularly in FE motion, the function of the PLC was considered to be incompetent.
Keywords: biomechanics, posterior ligamentous complex, sequential resection, thoracolumbar spine
1. Introduction
The posterior ligamentous complex (PLC) consists of the supraspinous ligament (SSL), interspinous ligament (ISL), facet capsule ligament (FCL), and ligamentum flavum (LF). It interconnects with the fascia and musculature, acting as an integrated unit. This complex resists bending and compressive loads to provide stability by limiting excessive motion. A PLC rupture leads to kyphosis and instability of the thoracolumbar fracture.[1–7] In addition, the integrality of the PLC is a serious factor related to stability evaluation and treatment selection for the thoracolumbar injury classification and severity score (TLICS).[8] Several studies have reported the mechanical role played by spinal ligaments in the stepwise transection of posterior spinal structures. However, most of this sequence spans from dorsal to ventral.[9–14]
Recently, a magnetic resonance imaging study reported that with increased traumatic forces, the PLC showed a progressive orderly rupture among the different PLC components. The ligament failure starts from the FCL, then moves to ISL involvement, extends to the SSL, and finally ends with a lacerated LF.[15] Additional, at the stage of facet capsule and ISL, without SSL or LF injury, the PLC is considered to be competent. Furthermore, with SSL or LF lesions, the PLC appears to be incompetent.[16] To our knowledge, no specific biomechanical experimental studies have validated this assumption.
We hypothesized that with SSL or LF disruption, the range of motion (ROM) and neutral zone (NZ) will be increased more significantly than with other ligament failures. Therefore, the purpose of this study was to quantify the increase in motion produced after a new stepwise resection based on the PLC injury sequence in simulated flexion–extension (FE), lateral bending (LB), and axial rotation (AR).
2. Materials and methods
This study was approved by the ethics committee of our institution (the Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University Research Ethics Committee Meeting).
2.1. Specimen preparation
A total of 12 fresh human thoracolumbar specimens (T11–L3) were harvested from the Department of Anatomy of the Wenzhou Medical University (Zhejiang, China). Each spinal specimen was assessed through plain film radiographs, dual energy X-ray absorptiometry scans, and available history to ensure that no specimen had osteoporosis, metastatic disease, or any major radiographic flaws. All specimens were wrapped in saline-soaked gauze, kept in double plastic bags, and stored frozen at −20°C. Before testing, the specimens were thawed at room temperature in a humidity-controlled circumstance for 8 to 12 h. Then, the muscle was removed without bone, discs, and spinal ligaments. The cranial (T11) and caudal (L3) from each specimen were mounted in Plexiglas casts. Specimen alignment was achieved with the middle vertebrae (L1) horizontally. A saline solution was sprayed to keep the spine moist during the test period. A previous biomechanical study has validated that the solution does not alter the material characteristics of the bone and soft tissues.[17]
2.2. Experimental protocol
Specimens were tested under 6 conditions:
-
(1)
Intact: An intact specimen includes the integrity and functionality of ligaments, facet capsules, and intervertebral discs. The intertransverse ligaments were missing and not considered in the presented study.
-
(2)
Fracture model: A controlled injury pattern was produced in the present study.[18] A 2-mm drill bit was used to create holes and to resect a 1/3 L1 vertebral body in a wedged shape. After the preinjury had been created, the specimens were mounted on a universal testing machine (MTS 858 Bionix with TestStar, MTS Systems Corporation, Eden Prairie, MN) and subjected to flexion to produce a compression fracture in the L1 vertebrae.
-
(3)
The PLC sequential disruption of the T12–L1 level was performed in the following order. FCL: the specimens were axially rotated, appreciating the joint gap for removal. In this study, the bilateral facet capsules were involved. ISL: along the fiber arrangement of the ISL from the inside to the outside. SSL: the SSL was dissected between the gap of the T12–L1 spinous processes. LF: along the gap of the T12–L1 lamina with slight flexion, then the LF was cut.
2.3. Kinematics test
The relative motion between the T12–L1 and L1–L2 vertebral bodies was measured. The loading simulator was applied to the cranial end (T11), and the caudal end (L3) was fixed to the bottom of the simulator. All specimens were subjected to 6 Nm to simulate spinal pure movements of FE, left/right LB, left/right AR. To avoid viscoelastic effects, each movement was repeated for 3 load–unload cycles and allowed to creep for 30 s at each load steps. The final cycle load was used for analysis.
Before the biomechanical test, fluorescent markers were inserted into each vertebral body for digital image reconstruction, and 4 fluorescent balls were placed in each segment to create a motional plane (Fig. 1). On the third load cycle, the stereo model of the specimen was created using 10 laser scanners (Eagle digital scanner, Motion Analysis Corporation, Santa, Rosa, CA) and stored on computer (Fig. 2). The marker coordinates were then digitized, and 6 degrees of freedom intervertebral motion was calculated with computer software (Cortex 4.0, Motion Analysis Corporation). The maximum error in marker position was 0.1 mm (1 degree segmental angle) for a 60 × 60 × 150 mm measuring space. Kinematics data were collected from the intact group and then the fracture model group at each stage of the PLC sequential disruptions. Measurements of interest included the ROM and NZ at the level of T12–L1 and L1–L2. The NZ for each movement was defined as the displacement from the neutral position on the 0-load point of the 3rd load cycle. This terminology was previously developed by Panjabi.[19]
Figure 1.
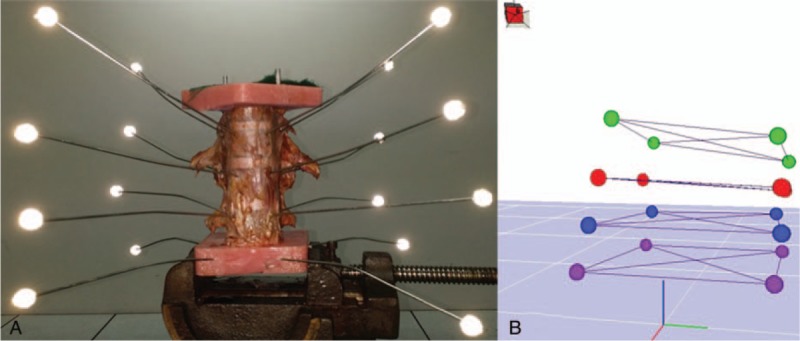
Experimental setup. Motion was applied to the thoracolumbar spine specimens (T11–L3). The cranial (T11) and caudal (L3) were mounted in Plexiglas casts. The caudal was fixed to the table vice, and the cranial was fixed to the loading jig. Axial rotation and flexion–extension were applied in the same direction. Lateral bending required a 90° rotation (A). To capture these motions, 4 fluorescent markers were inserted in each vertebral plane and a 3-dimensional model was reconstructed through computer software (B).
Figure 2.
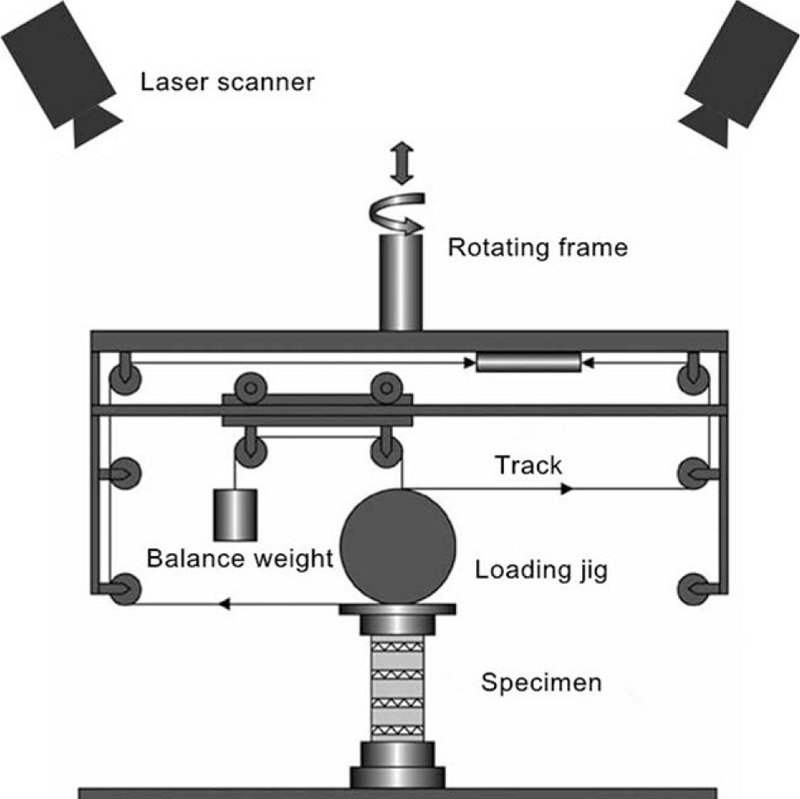
A diagram of the test machine and laser scanners for motion analysis. The loading jig was balanced with a counterweight. The 2 forces applied to the loading jig were parallel, opposite, and equal. Laser scanners connected with a computer used the fluorescent signal to create a 3-dimensional model.
2.4. Statistical analysis
Statistical analysis was performed using SPSS 16.0 software (SPSS/PC Inc, Chicago, IL). Kinematics data were evaluated using 1-way repeated measures ANOVA with a factor of injury stage alone, followed by Tukey post hoc test for pair-wise comparisons. A P value < .05 was considered to be significant.
3. Results
3.1. Included specimens
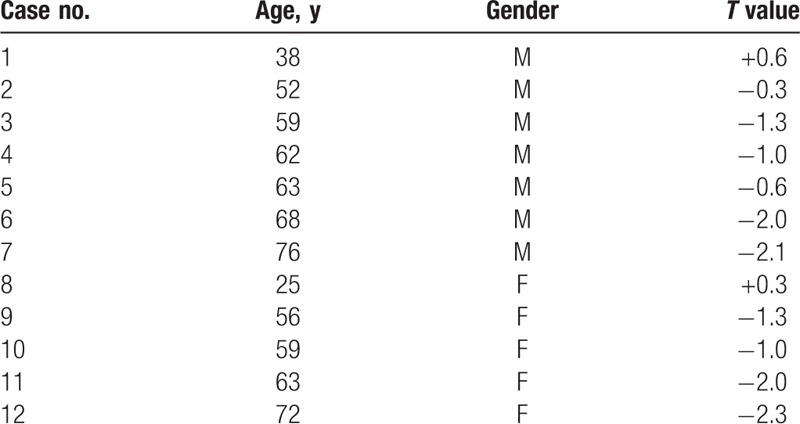
A total of 12 human specimens were included in the present study. The specimen demographics are presented in Table 1. There are 7 males and 5 females, and the mean age was 57.8 years (range 25–76 years). The mean T values was −1.1 (range −2.3 to +0.6).
Table 1.
Overview of specimens and their relevant characteristics.
3.2. T12–L1 segment
The mean values and standard deviations of T12–L1 for ROM and NZ are presented in Table 2. For ROM, there was an effect of the injury state on FE (P < .01), LB (P < .01), and AR (P = .023). In FE, ROM in the intact state was less than that of the fracture model (P < .01). There was no significant increase after resection of the FCL (P = .104) and ISL (P = .074). Compared with the fracture model, there was also no difference between the FCL and ISL resections (P = .906). Additional SSL transection resulted in a significant increase compared with the intact and previous injury stages (P < .05). After LF disruption, there was also a further increase over all other injury states (P < .01). For LB, the fracture model showed slight but significant increases compared with the intact stage (P < .01). FCL resection resulted in an increase compared with the fracture model (P < .01), and there was no difference between the FCL, ISL, and SSL resections (P > .05). The final LF transection showed a significant increase over the previous injury stages (P < .05). For AR, there was a slight increase after the fracture compared with the intact stage (P < .01). The LF cut resulted in a significant increase over the previous injury stages (P < .05).
Table 2.
Averaged ROM and NZ at T12–L1 (mean ± standard deviation, °).
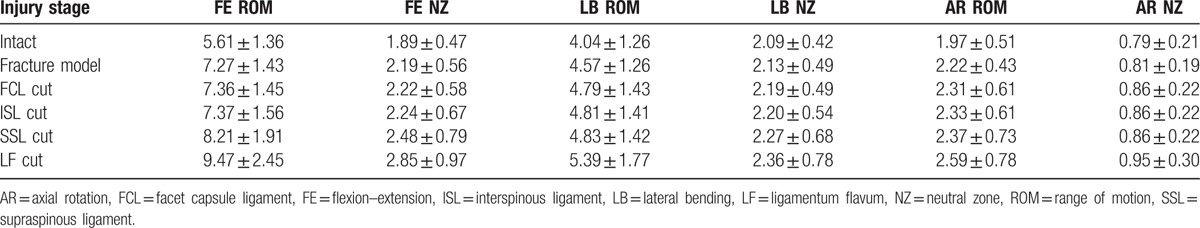
In terms of the NZ, the injury stage did affect FE (P < .01) and LB (P < .01). For FE, the NZ of the intact stage was less than that of the fracture model (P < .01), and in all other PLC injury stages (P < .05), transection of the FCL (P = .153) and ISL (P = .067) did not result in a significant increase compared with the fracture model. Subsequent SSL disruption showed an increase compared with the fracture model, FCL and ISL cuts (P < .01). Finally, the LF cut resulted in increases over all previous injuries (P < .05). For LB, the last LF resection increased the NZ compared with the intact stage (P = .013) and fracture model (P = .025). The injury stage did not affect AR (P = .076).
3.3. L1–L2 segment
The mean values and standard deviations of L1–L2 for ROM and NZ are shown in the Table 3. For both ROM and NZ, there was no effect of the injury stage in FE and AR (P > .05). In LB, there was a difference in ROM only (P < .01). The FCL cut resulted in a statistical increase compared with the intact (P < .01) and fracture model (P = .020).
Table 3.
Averaged ROM and NZ at L1–L2 (mean ± standard deviation, °).
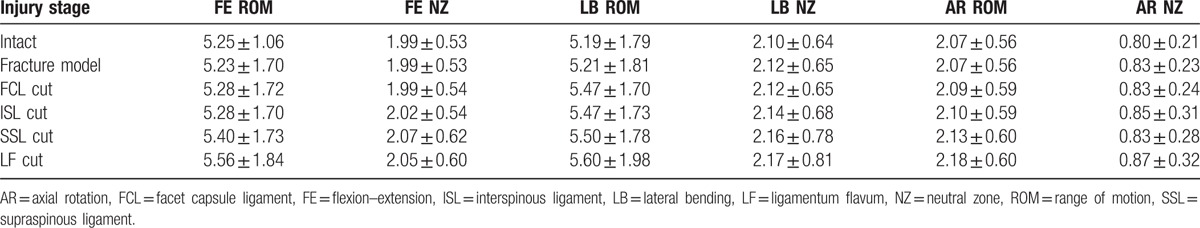
3.4. Percentage of ROM in the intact and fracture model
The percentage of ROM in the intact and fracture model in the progression injury stage are shown in Figs. 3 and 4, respectively. Only the ROM of the T12–L1 segment was considered. As a percentage of the intact stage in FE, the fracture model resulted in a 29.4% increase compared with the intact stage. Resection of the FCL and ISL presented no significant increases. There was a further increase (46.4%) after removal of the SSL compared with the intact stage. Finally, the LF cut led to a 68.9% increase over the intact model. For LB compared with the intact stage, and the fracture model, resection of FCL and LF resulted in 13.1%, 18.6%, and 33.6% increases, respectively. However, there was no significant increase for transection of the ISL and SSL. An increase in AR was caused by the fracture model (19.9%) and the LF cut (34.5%).
Figure 3.
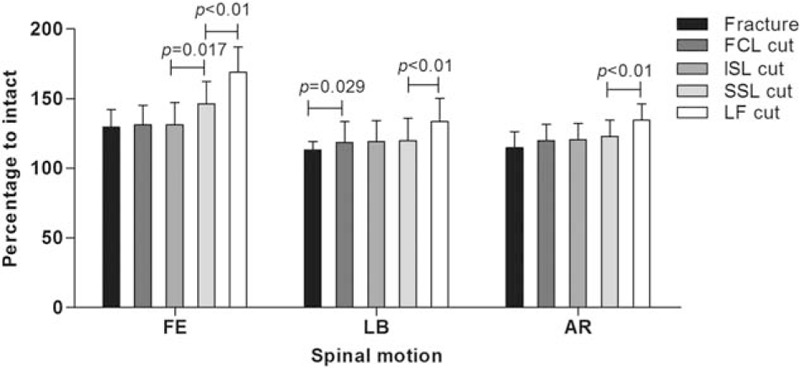
Percentage of range of motion to the intact stage for the fracture model and sequential resection of the posterior ligamentous complex in the T12–L1 segment.
Figure 4.
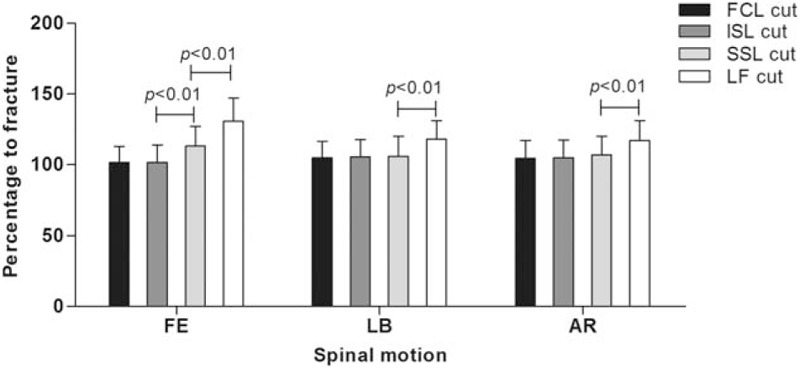
Percentage of range of motion to the fracture model for sequential resection of the posterior ligamentous complex in the T12–L1 segment.
As percentage of ROM to fracture model in FE, there was also no increase compared with the fracture model with resection of the FCL and ISL, resection of SSL led to a 13.1% increase, and finally the LF cut resulted in a 30.7% increase. For LB, compared with the fracture model, the last removal of LF caused an 18.0% increase. For AR, only resection of LF led to a significant increase (17.1%).
4. Discussion
The thoracolumbar spine has a special structure and biomechanics resulting in spinal fractures that primarily occur in the thoracolumbar region, and nearly 20% of these fractures are combined with PLC failure. The importance of this complex has been recognized in maintaining the stability of the thoracolumbar spine, and disruption of the PLC may imply a relative instability of the spine and surgery intervention. Vaccaro et al[8] developed the TLICS, which proposed grading spinal stability based on injury morphology, PLC integrity, and neurologic status. The integrity of the PLC contributes 3 of a total of 10 points to this algorithmic approach for surgical decision making. Therefore, our study was designed to establish the biomechanical contribution of the different PLC components and to define whether there is a boundary between competent and incompetent PLC.
Several studies were performed to determine the PLC contribution to spinal stability through stepwise resection of the ligaments on specimens. Wilke et al[20] produced a spinal instability model by cutting the bilateral facet joint, the SSL and ISL ligament, and LF, which increased the ROM by approximately 8% in LB and 18% in AR. Gillespie and Dickey[10] adopted sequential removal of constituent of the PLC from outside to inside. They found that the supraspinous ISL complex contributed 35.9% to resistance of the peak flexion moment. A previous study by Heuer et al[12] investigated stepwise reduction of spinal posterior structures. The ROM and lordosis angle of the L4–5 segment increased with each increase of the defect situation.
Most previous biomechanical studies have reported stepwise removal of the PLC from the SSL to the ISL, then the FCL, and finally the LF. However, there has been controversy concerning the sequence of the PLC disruption. Adams et al[21] observed that the ISL–SSL ligament complex is an inflexion point and the first to be injured in flexion. However, some studies have shown that the fibers of ISL were arranged in an S shape when the ligament relaxed, and it did start functioning until halfway through the flexion movement. In addition, Pizones et al[15] analyzed the association between magnetic resonance imaging signal of the PLC injury and the arbeitsgemeinschaftfür osteosynthesefragen progression of morphological damage. They reported that the PLC components demonstrated an orderly rupture sequence as the traumatic force increased, the posterior distraction forces cause capsule distraction, then injure the ISL, and the SSL becomes involved, finally damaging the LF. In their subsequent study, they proposed that the rupture of the SSL acts as the key point leading to PLC incompetence.[16]
In spinal biomechanical study, there are many methods of creating spinal models, most which include the following: a free-falling weight impact fracture, the impact method aligns better with the clinical situation but with poor repeatability.[22–24] Partial or total vertebral structure removal, compared with the impact method, the resection method is considered a simple operation, with better repeatability and controllable severity of injury model; however, it does not actually imitate the clinical injury.[5,25] A preinjury model with a testing machine or weight drop to used to create a fracture.[26,27] In the model presented here, different spinal structures are removed to imitate 2 or 3 column spinal injury models, according to the 3-column theory of Denis, and then they are mounted on a universal testing machine to create an injury model. This is a viable option for biomechanical study of the spine. In our study, we remove 1/3 L1 vertebral body in a wedged shape, after the preinjury had been created. The specimens were subjected to flexion–compression to create a fracture model. This method can be used to create anterior-middle spinal column injury and controls the extent and part of the spinal fracture. Comparing the intact stage with the fracture model, the spinal stability declined significantly in the presented study.
We refer to a progressive orderly rupture sequence of the different components related to an anterior column injury of the spine. The present study showed the biomechanical effects of stepwise reduction of the PLC on the stability of the injury thoracolumbar spine. With sequenced removal of the PLC, the ROM and NZ presented an increase tendency. In addition, SSL resection resulted in a significant increase in motion toward flexion. Although the degree of the NZ was relatively small, statistical analysis showed that the increases were significant. The data also demonstrate a significantly increase for LF failure in FE, LB, and AR. Currently, the treatment choice primarily depends on spinal stability. Our findings will be more helpful for clinical studies. Before the SSL or LF lesion, this injury stage implies PLC competence. With SSL or LF rupture, the function of the PLC should be considered incompetent and unstable. This finding suggests a need for posterior approach fixation to reconstruct posterior tension bands. Furthermore, this finding may deepen the TLICS. If the PLC is considered to be incompetent, then the assessment scores of the PLC should be increased.
However, some limitations of the present study should be noted. First, because human specimens are so uncommon, there were some variations in our study, such as age, gender, soft tissue quality, and disc degeneration. On the other hand, the different fracture types existed in injured thoracolumbar spines, including flexion (compression and axial burst fracture), extension, and rotation fracture pattern. The authors chose to investigate only the compression fracture type, which was produced by preinjured, followed by a compressed lesion, this method resulted in a constant and controllable in injury severity. Furthermore, the third limitation of the current investigation is that the in vivo environment differs from the in vitro environment, correlating to the lack of muscle attachments and tissue fatigue in a repeated manner.
In conclusion, our investigation provides insight into the mechanical role of the stability of the thoracolumbar spine. Stepwise resection of PLC of the T12–L1 resulted in an increase in ROM and NZ. With rupture of the SSL or LF, the stability of the T12–L1 segment decreased sharply, particularly in FE motion. The function of the PLC is considered to be incompetent. The SSL and LF were the critical components of the PLC needed to maintain the stability of the compression thoracolumbar spine.
Footnotes
Abbreviations: AR = axial rotation, FCL = facet capsule ligament, FE = flexion–extension, ISL = interspinous ligament, LB = lateral bending, LF = ligamentum flavum, NZ = neutral zone, PLC = posterior ligamentous complex, ROM = range of motion, SSL = supraspinous ligament, TLICS = thoracolumbar injury classification and severity score.
This work was supported by grants from the Natural Science Foundation of Zhejiang Province for Distinguished Young Scholars (Grant No. LR12H060001) and the Major Science and Technology Program for Medical and Health of Zhejiang Province (Grant No. WKJ-ZJ-1527).
The authors have no conflicts of interest to disclose.
References
- [1].Behrsin JF, Briggs CA. Ligaments of the lumbar spine: a review. Surg Radiol Anat 1988;10:211–9. [DOI] [PubMed] [Google Scholar]
- [2].Johnson GM, Zhang M. Regional differences within the human supraspinous and interspinous ligaments: a sheet plastination study. Eur Spine J 2002;11:382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].McAfee PC, Yuan HA, Fredrickson BE, et al. The value of computed tomography in thoracolumbar fractures. An analysis of one hundred consecutive cases and a new classification. J Bone Joint Surg Am 1983;65:461–73. [PubMed] [Google Scholar]
- [4].Oner FC, van Gils AP, Faber JA, et al. Some complications of common treatment schemes of thoracolumbar spine fractures can be predicted with magnetic resonance imaging: prospective study of 53 patients with 71 fractures. Spine 2002;27:629–36. [DOI] [PubMed] [Google Scholar]
- [5].Panjabi MM, Hoffman H, Kato Y, et al. Superiority of incremental trauma approach in experimental burst fracture studies. Clin Biomech 2000;15:73–8. [DOI] [PubMed] [Google Scholar]
- [6].Radcliff K, Su BW, Kepler CK, et al. Correlation of posterior ligamentous complex injury and neurological injury to loss of vertebral body height, kyphosis, and canal compromise. Spine 2012;37:1142–50. [DOI] [PubMed] [Google Scholar]
- [7].Vaccaro AR, Lee JY, Schweitzer KM, Jr, et al. Assessment of injury to the posterior ligamentous complex in thoracolumbar spine trauma. Spine J 2006;6:524–8. [DOI] [PubMed] [Google Scholar]
- [8].Vaccaro AR, Lehman RA, Jr, Hurlbert RJ, et al. A new classification of thoracolumbar injuries: the importance of injury morphology, the integrity of the posterior ligamentous complex, and neurologic status. Spine 2005;30:2325–33. [DOI] [PubMed] [Google Scholar]
- [9].Alapan Y, Demir C, Kaner T, et al. Instantaneous center of rotation behavior of the lumbar spine with ligament failure. J Neurosurg Spine 2013;18:617–26. [DOI] [PubMed] [Google Scholar]
- [10].Gillespie KA, Dickey JP. Biomechanical role of lumbar spine ligaments in flexion and extension: determination using a parallel linkage robot and a porcine model. Spine 2004;29:1208–16. [DOI] [PubMed] [Google Scholar]
- [11].Heuer F, Schmidt H, Claes L, et al. Stepwise reduction of functional spinal structures increase vertebral translation and intradiscal pressure. J Biomech 2007;40:795–803. [DOI] [PubMed] [Google Scholar]
- [12].Heuer F, Schmidt H, Klezl Z, et al. Stepwise reduction of functional spinal structures increase range of motion and change lordosis angle. J Biomech 2007;40:271–80. [DOI] [PubMed] [Google Scholar]
- [13].Heuer F, Schmidt H, Wilke HJ. Stepwise reduction of functional spinal structures increase disc bulge and surface strains. J Biomech 2008;41:1953–60. [DOI] [PubMed] [Google Scholar]
- [14].Rasoulinejad P, McLachlin SD, Bailey SI, et al. The importance of the posterior osteoligamentous complex to subaxial cervical spine stability in relation to a unilateral facet injury. Spine J 2012;12:590–5. [DOI] [PubMed] [Google Scholar]
- [15].Pizones J, Izquierdo E, Sanchez-Mariscal F, et al. Sequential damage assessment of the different components of the posterior ligamentous complex after magnetic resonance imaging interpretation: prospective study 74 traumatic fractures. Spine 2012;37:E662–7. [DOI] [PubMed] [Google Scholar]
- [16].Pizones J, Zuniga L, Sanchez-Mariscal F, et al. MRI study of post-traumatic incompetence of posterior ligamentous complex: importance of the supraspinous ligament. Prospective study of 74 traumatic fractures. Eur Spine J 2012;21:2222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wilke HJ, Wenger K, Claes L. Testing criteria for spinal implants: recommendations for the standardization of in vitro stability testing of spinal implants. Eur Spine J 1998;7:148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang XY, Dai LY, Xu HZ, et al. Biomechanical effect of the extent of vertebral body fracture on the thoracolumbar spine with pedicle screw fixation: an in vitro study. J Clin Neurosci 2008;15:286–90. [DOI] [PubMed] [Google Scholar]
- [19].Panjabi MM. Biomechanical evaluation of spinal fixation devices: I. A conceptual framework. Spine 1988;13:1129–34. [DOI] [PubMed] [Google Scholar]
- [20].Wilke HJ, Drumm J, Haussler K, et al. Biomechanical effect of different lumbar interspinous implants on flexibility and intradiscal pressure. Eur Spine J 2008;17:1049–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Adams MA, Hutton WC, Stott JR. The resistance to flexion of the lumbar intervertebral joint. Spine 1980;5:245–53. [DOI] [PubMed] [Google Scholar]
- [22].An HS, Singh K, Vaccaro AR, et al. Biomechanical evaluation of contemporary posterior spinal internal fixation configurations in an unstable burst-fracture calf spine model: special references of hook configurations and pedicle screws. Spine 2004;29:257–62. [DOI] [PubMed] [Google Scholar]
- [23].Chen HH, Wang WK, Li KC, et al. Biomechanical effects of the body augmenter for reconstruction of the vertebral body. Spine 2004;29:E382–7. [DOI] [PubMed] [Google Scholar]
- [24].Schreiber U, Bence T, Grupp T, et al. Is a single anterolateral screw-plate fixation sufficient for the treatment of spinal fractures in the thoracolumbar junction? A biomechanical in vitro investigation. Eur Spine J 2005;14:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wilcox RK, Allen DJ, Hall RM, et al. A dynamic investigation of the burst fracture process using a combined experimental and finite element approach. Eur Spine J 2004;13:481–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lu WW, Cheung KM, Li YW, et al. Bioactive bone cement as a principal fixture for spinal burst fracture: an in vitro biomechanical and morphologic study. Spine 2001;26:2684–90. [DOI] [PubMed] [Google Scholar]
- [27].Smit TH. The use of a quadruped as an in vivo model for the study of the spine—biomechanical considerations. Eur Spine J 2002;11:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]


