Figure 2. Two models of sexual differentiation.
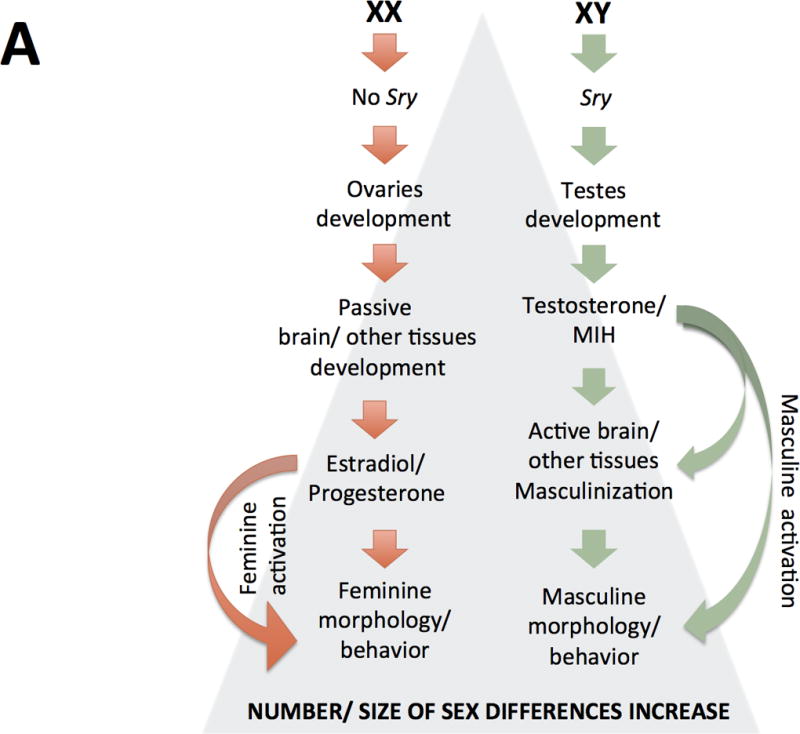
According to traditional views of sexual differentiation (panel A) XX and XY individuals would be basically indistinct till the gonadal expression of the Y-linked Sry gene Sry expression would lead the development of previously undifferentiated gonads as testes or, in females, the lack of Sry would lead to ovary development of ovaries. In the first case, testes’ secretions (mainly testosterone and Müller inhibiting hormone, MIH) or their metabolites acting at a critical prenatal period would differentially organize in males several organs and tissues, such as the brain, then changing their “by default” organization (that observed in females); these structural sex-differences would remain largely silent till puberty, when a second (and more or less permanent) rise of gonadal steroid hormones would set them in motion, producing further sex-differences in physiology and behavior. On the other hand, if gonads develop as ovaries, no immediate secretions are produced and, in absence of “masculinizing” signaling, leading to the passive development of organs and tissues including the brain. Those feminine-differentiated tissues are activated by the raise of estradiol and progesterone from the ovaries.
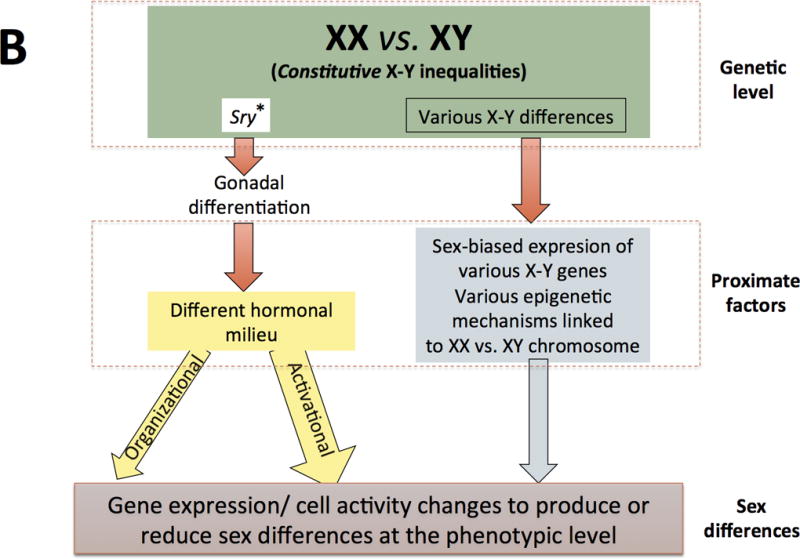
In contrast, current views of sexual differentiation (panel B) pose unequal chromosome complement as primary sources of physiological disparity between males and females, some of which occur before gonadal differentiation. Sry is still considered a major agent of gonadal differentiation, but it is known that the expression (i.e. Sox9) and repression (i.e.FoxL2) of other genes is needed for testes development. Similarly, the lack of Sry and the sustained expression of FoxL2 and other genes as well as other currently less known mechanisms drive the also active ovaries’ differentiation. Gonadal differentiation into ovaries or testicles leads to different hormonal milieus in females and males, resulting in hormonal organizing and activating (more common) actions at several tissues, including the brain. Within those tissues, chromosomal complement and gonadal steroids act as synergistic or antagonistic proximal factors leading to gene expression changes and other cellular modifications that finally to produce or reduce phenotypic differences between females and males. Some of these differences are due to the presence of two X chromosomes in females and subsequent X-inactivation (or escape from inactivation), other differences are due the presence of genes on the Y chromosome not present on the X chromosome and still other differences are due to other chromosomic complement inequalities (figure 3).
It should be noted that, current views of sexual differentiation have left behind the idea of a “default” or “passive” development of females. On the other hand, as compared to its “traditional” conceptualization, organizational and activational actions of steroids are not longer so rigidly separated and they are currently conceived in a wider sense (see main text). A third major difference between both models (but not properly illustrated in these schemes) is that newer views do not categorize all (not even most) sexually-biased outcomes as binary but rather as being expressed as a continuous.
(These figures are adapted from Arnold AP (2012) The end of gonad-centric sex determination in mammals. Trends Genet 28: 55–61).
