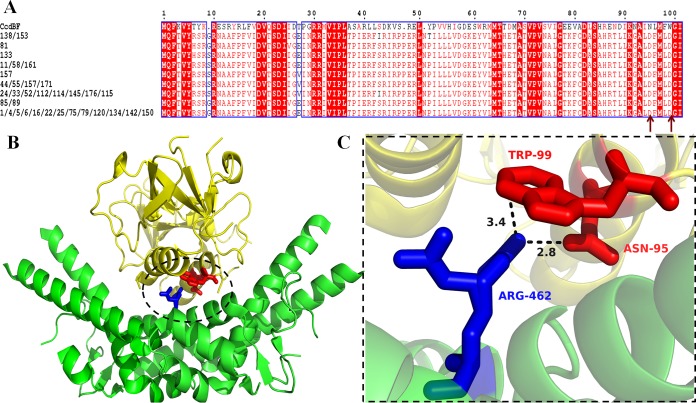
FIG 6.
Sequence and structural basis for differential activity of CcdBF and CcdBO157. (A) Sequence alignment of CcdBF and CcdBO157 serotypes. Sequenced variants of CcdBO157 from 32 different isolates, which tested positive for toxicity (12), were aligned with the CcdBF sequence using the Clustal Omega multiple-sequence alignment tool. Residues that are fully conserved either are active-site residues or form a part of the hydrophobic core (34). (B) CcdBF-gyrase A14 bound crystal structure (PDB no. 1X75) (40). The key region of the interaction between CcdBF and gyrase A14 is circled. (C) Residues in the interaction between CcdB (in red) and gyrase A14 (in blue) that are not identical in the CcdBF and CcdBO157 sequences (marked with arrows in the multiple-sequence alignment in panel A). CcdBO157 contains the mutations N95D and W99D relative to CcdF.