Fig. 8.
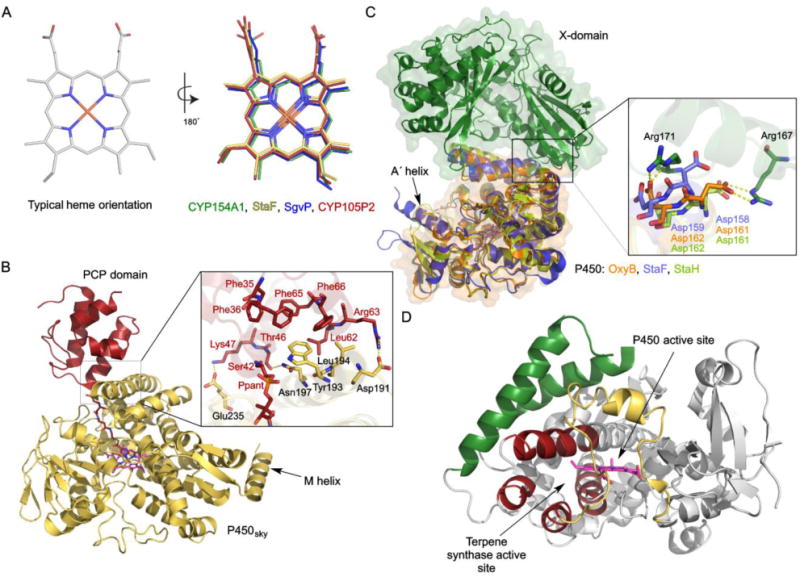
Selected variations in the structures of P450s from Streptomyces. (A) Typical heme orientations and inversed orientations in four different P450s (PDB IDs: 1ODO, 5EX8, 4MM0, 5IT1). (B) The crystal structure of P450sky in complex with an inhibitor-tethered PCP domain (PDB ID: 4PXH). The inset shows a zoomed-in look at the interface of P450sky and the PCP domain highlighting the hydrophobic and electrostatic interactions. The unusual C-terminal M-helix is labeled. (C) Structural superposition of StaF (PDB ID: 5EX8) and StaH (PDB ID: 5EX6) with OxyB in complex with the X-domain (PDB ID: 4TX3). The inset shows the two Asp residues from the conserved PRDD motif, which are proposed to be involved in recruitment by the X-domain via interaction with two Arg residues from the X-domain. (D) The crystal structure of CYP170A1 (PDB ID: 3EL3). The B–C loop and F–G region, colored in yellow and green, respectively, form the P450 active site. The four helices, colored in red, form the terpene synthase active site.
