Fig. 9.
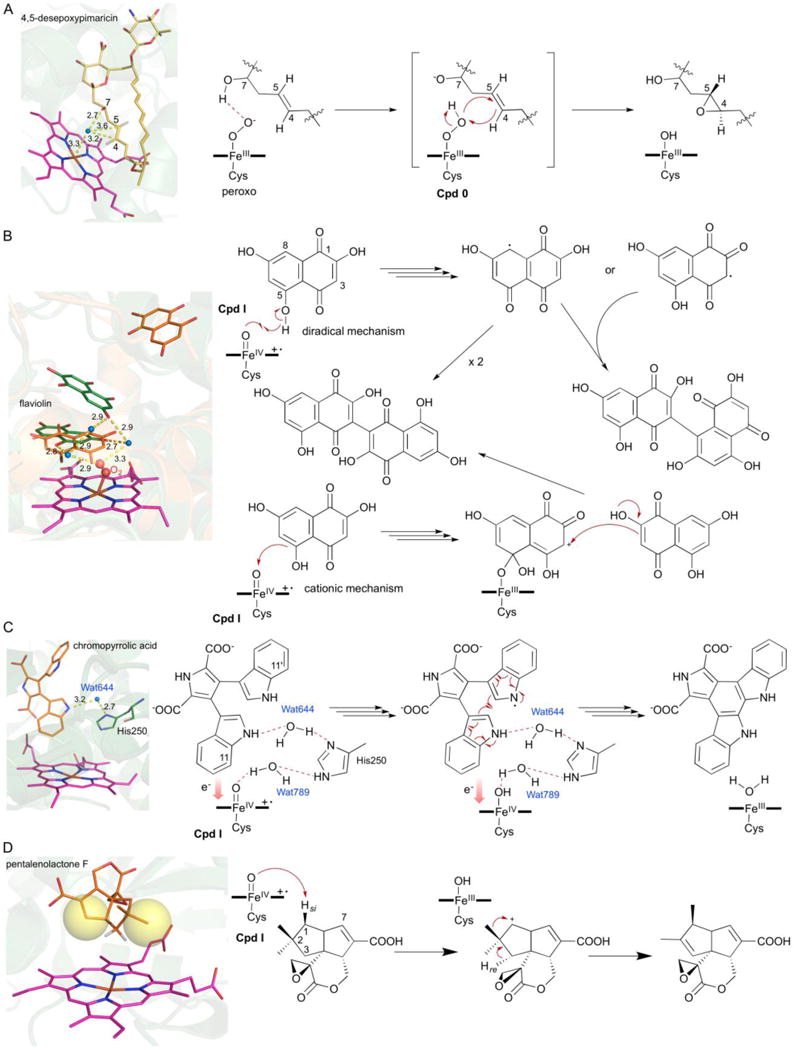
Structure-based mechanistic studies of P450s from Streptomyces. (A) Epoxidation by PimD via the hydroperoxyferric intermediate, Cpd 0 (PDB ID: 2XBK). (B) Substrate-assisted biaryl ring coupling by CYP158A1 and CYP158A2 (PDB IDs: 2NZ5 and 2D09). The two different binding modes of the two biflaviolin substrates are shown in orange and green. (C) Intramolecular biaryl ring coupling by StaP (PDB IDs: 2Z3U). Wat644 and His250 are shown in the active site; Wat789 is liberated during the formation of Cpd I (Wang 2009). (D) Oxidative rearrangement by PntM via a carbocation intermediate (PDB IDs: 5L1O). Blue dots in each figure represent water molecules; yellow spheres depict steric hindrance.
